
In chemistry, cyanide is a chemical compound that contains a C≡N functional group. This group, known as the cyano group, consists of a carbon atom triple-bonded to a nitrogen atom.

Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as halides of carbon without carbon-hydrogen and carbon-carbon bonds, and certain compounds of carbon with nitrogen and oxygen.
Hydrogen cyanide is a chemical compound with the formula HCN and structural formula H−C≡N. It is a highly toxic and flammable liquid that boils slightly above room temperature, at 25.6 °C (78.1 °F). HCN is produced on an industrial scale and is a highly valued precursor to many chemical compounds ranging from polymers to pharmaceuticals. Large-scale applications are for the production of potassium cyanide and adiponitrile, used in mining and plastics, respectively. It is more toxic than solid cyanide compounds due to its volatile nature. A solution of hydrogen cyanide in water, represented as HCN, is called hydrocyanic acid. The salts of the cyanide anion are known as cyanides.

Sodium cyanide is a compound with the formula NaCN and the structure Na+−C≡N. It is a white, water-soluble solid. Cyanide has a high affinity for metals, which leads to the high toxicity of this salt. Its main application, in gold mining, also exploits its high reactivity toward metals. It is a moderately strong base.
Cyanogen is the chemical compound with the formula (CN)2. The simplest stable carbon nitride, it is a colorless and highly toxic gas with a pungent odor. The molecule is a pseudohalogen. Cyanogen molecules consist of two CN groups ‒ analogous to diatomic halogen molecules, such as Cl2, but far less oxidizing. The two cyano groups are bonded together at their carbon atoms: N≡C‒C≡N, though other isomers have been detected. The name is also used for the CN radical, and hence is used for compounds such as cyanogen bromide (NCBr) (but see also Cyano radical). When burned at increased pressure with oxygen, it is possible to get a blue tinted flame, the temperature of which is about 4800°C (a higher temperature is possible with ozone). It is as such regarded as the gas with the second highest temperature of burning (after dicyanoacetylene).

Potassium cyanide is a compound with the formula KCN. It is a colorless salt, similar in appearance to sugar, that is highly soluble in water. Most KCN is used in gold mining, organic synthesis, and electroplating. Smaller applications include jewellery for chemical gilding and buffing. Potassium cyanide is highly toxic, and a dose of 200 to 300 milligrams will kill nearly any human.
The Leuchter report is a pseudoscientific document authored by American execution technician Fred A. Leuchter, who was commissioned by Ernst Zündel to defend him at his trial in Canada for distributing Holocaust denial material. Leuchter compiled the report in 1988 with the intention of investigating the feasibility of mass homicidal gassings at Nazi extermination camps, specifically at Auschwitz. He traveled to the camp, collected multiple pieces of brick from the remains of the crematoria and gas chambers, brought them back to the United States, and submitted them for chemical analysis. At the trial, Leuchter was called upon to defend the report in the capacity of an expert witness; however, during the trial, the court ruled that he had neither the qualifications nor experience to act as such.
A blood agent is a toxic chemical agent that affects the body by being absorbed into the blood. Blood agents are fast-acting, potentially lethal poisons that typically manifest at room temperature as volatile colorless gases with a faint odor. They are either cyanide- or arsenic-based.

The Death Dealers is a 1958 mystery novel by American writer Isaac Asimov. It is about a university professor whose research student dies while conducting an experiment. The professor attempts to determine if the death was accident, suicide or murder.

Peroxymonosulfuric acid, also known as persulfuric acid, peroxysulfuric acid is the inorganic compound with the formula H2SO5. It is a white solid. It is a component of Caro's acid, which is a solution of peroxymonosulfuric acid in sulfuric acid containing small amounts of water. Peroxymonosulfuric acid is a very strong oxidant.
Cyanogen bromide is the inorganic compound with the formula (CN)Br or BrCN. It is a colorless solid that is widely used to modify biopolymers, fragment proteins and peptides, and synthesize other compounds. The compound is classified as a pseudohalogen.
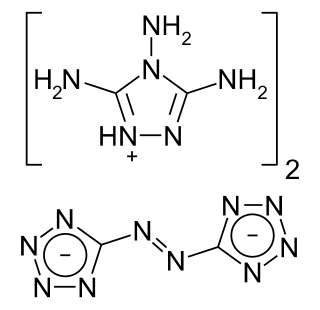
G2ZT is a bistetrazole. It is an explosive approximately as powerful as RDX, but it releases less toxic reaction products when detonated: ammonia and hydrogen cyanide. When combined with ADN or AN oxidizers, the amount of HCN produced by a deflagration may be reduced. The compound is thus considered by its advocates to be an environmentally friendlier explosive than traditional nitroamine-based explosives.

Cadmium cyanide is an inorganic compound with the formula Cd(CN)2. It is a white crystalline compound that is used in electroplating. It is very toxic, along with other cadmium and cyanide compounds.

Calcium cyanide is the inorganic compound with the formula Ca(CN)2. It is the calcium salt derived from hydrocyanic acid. It is a white solid, although the pure material is rarely encountered. It slowly hydrolyses in solution or moist air to release hydrogen cyanide and is very toxic.

Lithium cyanide is an inorganic compound with the chemical formula LiCN. It is a toxic, white coloured, hygroscopic, water-soluble salt that finds only niche uses.

Barium cyanide is a chemical compound with the formula Ba(CN)2. It is synthesized by the reaction of hydrogen cyanide and barium hydroxide in water or petroleum ether. It is a white crystalline salt.

Trifluoroacetyl chloride (also known as TFAC) is a toxic gaseous chemical compound with the chemical formula C2ClF3O. TFAC is the perfluorinated version of acetyl chloride. The compound is a gas, but it is usually shipped as a liquid under high pressure.
Aluminium cyanide is a metallic cyanide with a chemical formula of Al(CN)3. It is a white solid that undergoes hydrolysis to produce aluminium hydroxide and hydrogen cyanide.

Cacodyl cyanide is a highly toxic organoarsenic compound discovered by Robert Bunsen in the 1840s. It is very volatile and flammable, as it shares the chemical properties of both arsenic and cyanide.
Magnesium cyanide is a chemical compound with the formula Mg(CN)2. It is a toxic white solid. Unlike calcium isocyanide, the cyanide ligands prefer to coordinate at carbon, with a 0.3‑kcal/mol isomerization barrier. When this salt is heated to 500 °C, it decomposes to magnesium nitride.














