In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline; see Category:Amines for a list of amines. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.

In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)–. The simplest ketone is acetone, with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.

In organic chemistry, an aldehyde is an organic compound containing a functional group with the structure R−CH=O. The functional group itself can be referred to as an aldehyde but can also be classified as a formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
Hydroboration–oxidation reaction is a two-step hydration reaction that converts an alkene into an alcohol. The process results in the syn addition of a hydrogen and a hydroxyl group where the double bond had been. Hydroboration–oxidation is an anti-Markovnikov reaction, with the hydroxyl group attaching to the less-substituted carbon. The reaction thus provides a more stereospecific and complementary regiochemical alternative to other hydration reactions such as acid-catalyzed addition and the oxymercuration–reduction process. The reaction was first reported by Herbert C. Brown in the late 1950s and it was recognized in his receiving the Nobel Prize in Chemistry in 1979.

In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.

Hydrazones are a class of organic compounds with the structure R1R2C=N−NH2. They are related to ketones and aldehydes by the replacement of the oxygen =O with the =N−NH2 functional group. They are formed usually by the action of hydrazine on ketones or aldehydes.
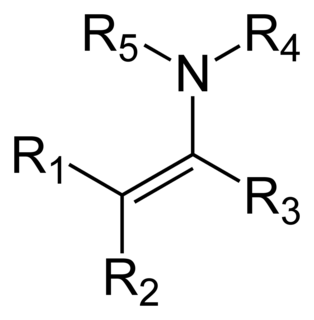
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
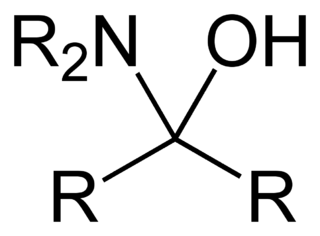
In organic chemistry, a hemiaminal is a functional group or type of chemical compound that has a hydroxyl group and an amine attached to the same carbon atom: −C(OH)(NR2)−. R can be hydrogen or an alkyl group. Hemiaminals are intermediates in imine formation from an amine and a carbonyl by alkylimino-de-oxo-bisubstitution. Hemiaminals can be viewed as a blend of aminals and geminal diol. They are a special case of amino alcohols.
In chemistry, a derivative is a compound that is derived from a similar compound by a chemical reaction.
Reductive amination is a form of amination that involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is considered the most important way to make amines, and a majority of amines made in the pharmaceutical industry are made this way.

In organic chemistry, an iminium cation is a polyatomic ion with the general structure [R1R2C=NR3R4]+. They are common in synthetic chemistry and biology.

Semicarbazide is the chemical compound with the formula OC(NH2)(N2H3). It is a water-soluble white solid. It is a derivative of urea.

Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless water-soluble liquid is a common precursor in organic chemistry and used in the industrial production of many pharmaceuticals. The hydrochloride salt was used to treat motion sickness on the Mercury-Atlas 6 mission in which NASA astronaut John Glenn became the first American to orbit the Earth.
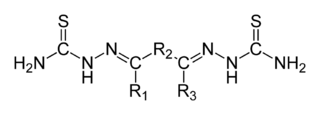
In organic chemistry, a bisthiosemicarbazone is a derivative from an elimination reaction between a thiosemicarbazide and a diketone. Their structure is H2NHC(=S)NN=C(R1)−R2−C(R3)=NNHC(=S)NH2. A 'thiosemicarbazone' contains a sulfur atom in lieu of the ketonic oxygen in semicarbazone. Bisthiosemicarbazones are known to have antiviral, antimalarial and anticancer activity, usually mediated through binding to copper or iron in cells. They have also been identified as potential ligands for radioisotope delivery, with selectivity towards hypoxic tissues, particularly in the heart and brain. When chelated to zinc atoms some bisthiosemicarbazones may have uses as fluorescing agents in optical microscopy.
The Baylis–Hillman reaction is a carbon-carbon bond forming reaction between the α-position of an activated alkene and a carbon electrophile such as an aldehyde. Employing a nucleophilic catalyst, such as a tertiary amine and phosphine, this reaction provides a densely functionalized product. It is named for Anthony B. Baylis and Melville E. D. Hillman, two of the chemists who developed this reaction while working at Celanese. This reaction is also known as the Morita–Baylis–Hillman reaction or MBH reaction, as K. Morita had published earlier work on it.

N-Sulfinyl imines are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display useful stereoselectivity reactivity and due to the presence of the chiral electron withdrawing N-sulfinyl group. They allow 1,2-addition of organometallic reagents to imines. The N-sulfinyl group exerts powerful and predictable stereodirecting effects resulting in high levels of asymmetric induction. Racemization of the newly created carbon-nitrogen stereo center is prevented because anions are stabilized at nitrogen. The sulfinyl chiral auxiliary is readily removed by simple acid hydrolysis. The addition of organometallic reagents to N-sulfinyl imines is the most reliable and versatile method for the asymmetric synthesis of amine derivatives. These building blocks have been employed in the asymmetric synthesis of numerous biologically active compounds.














