
In organic chemistry, ethers are a class of compounds that contain an ether group—a single oxygen atom bonded to two separate carbon atoms, each part of an organyl group. They have the general formula R−O−R′, where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether". Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

In chemistry, an ester is a compound derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. These compounds contain a distinctive functional group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species replaces a functional group within another electron-deficient molecule. The molecule that contains the electrophile and the leaving functional group is called the substrate.

An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism.
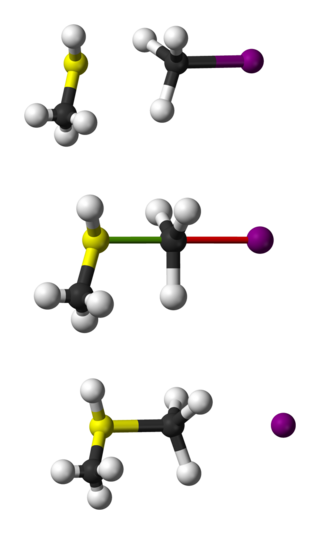
The bimolecular nucleophilic substitution (SN2) is a type of reaction mechanism that is common in organic chemistry. In the SN2 reaction, a strong nucleophile forms a new bond to an sp3-hybridised carbon atom via a backside attack, all while the leaving group detaches from the reaction center in a concerted fashion.
The Friedel–Crafts reactions are a set of reactions developed by Charles Friedel and James Crafts in 1877 to attach substituents to an aromatic ring. Friedel–Crafts reactions are of two main types: alkylation reactions and acylation reactions. Both proceed by electrophilic aromatic substitution.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.
In organic chemistry, an acyl chloride is an organic compound with the functional group −C(=O)Cl. Their formula is usually written R−COCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds that contain one or more carbon–chlorine bonds. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.

The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using carbon tetraiodide, methyl iodide or iodine gives alkyl iodides. The reaction is credited to and named after Rolf Appel, it had however been described earlier. The use of this reaction is becoming less common, due to carbon tetrachloride being restricted under the Montreal protocol.

Grignard reagents or Grignard compounds are chemical compounds with the general formula R−Mg−X, where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride Cl−Mg−CH3 and phenylmagnesium bromide (C6H5)−Mg−Br. They are a subclass of the organomagnesium compounds.

Organomercury chemistry refers to the study of organometallic compounds that contain mercury. Many organomercury compounds are highly toxic, but some are used in medicine, e.g., merbromin ("Mercurochrome") and the vaccine preservative thiomersal.

Allyl bromide (3-bromopropene) is an organic halide. It is an alkylating agent used in synthesis of polymers, pharmaceuticals, perfumes and other organic compounds. Allyl bromide is a colorless liquid, although commercial samples appear yellow or brown. It is an irritant and a potentially dangerous alkylating agent. Allyl bromide is more reactive but more expensive than allyl chloride, and these considerations guide its use.
Silylation is the introduction of one or more (usually) substituted silyl groups (R3Si) to a molecule. Silylations are core methods for production of organosilicon chemistry. Silanization, while similar to silylation, usually refers to attachment of silyl groups to solids. Silyl groups are commonly used for: alcohol protection, enolate trapping, gas chromatography, electron-impact mass spectrometry (EI-MS), and coordinating with metal complexes.
Amine alkylation (amino-dehalogenation) is a type of organic reaction between an alkyl halide and ammonia or an amine. The reaction is called nucleophilic aliphatic substitution, and the reaction product is a higher substituted amine. The method is widely used in the laboratory, but less so industrially, where alcohols are often preferred alkylating agents.

Carbonyl α-substitution reactions occur at the position next to the carbonyl group, the α-position, and involves the substitution of an α-hydrogen by an electrophile through either an enol or enolate ion intermediate.
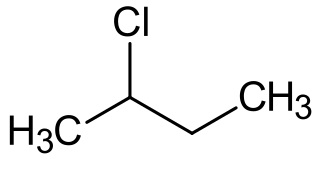
2-Chlorobutane is a compound with formula C4H9Cl. It is also called sec-butyl chloride. It is a colorless, volatile liquid at room temperature that is not miscible in water.
Ether cleavage refers to chemical substitution reactions that lead to the cleavage of ethers. Due to the high chemical stability of ethers, the cleavage of the C-O bond is uncommon in the absence of specialized reagents or under extreme conditions.
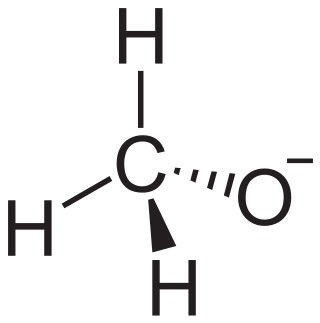
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as RO−, where R is the organyl substituent. Alkoxides are strong bases and, when R is not bulky, good nucleophiles and good ligands. Alkoxides, although generally not stable in protic solvents such as water, occur widely as intermediates in various reactions, including the Williamson ether synthesis. Transition metal alkoxides are widely used for coatings and as catalysts.
















