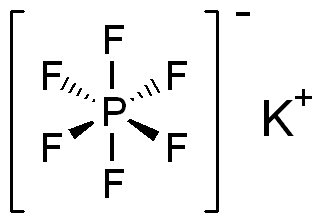In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions.
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved.
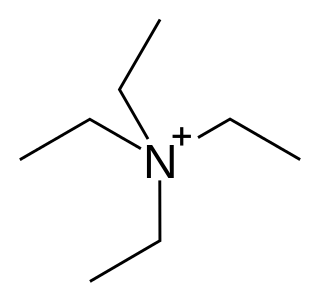
Tetraethylammonium (TEA), (NEt+
4) or (Et4N+) is a quaternary ammonium cation consisting of four ethyl groups attached to a central nitrogen atom, and is positively charged. It is a counterion used in the research laboratory to prepare lipophilic salts of inorganic anions. It is used similarly to tetrabutylammonium, the difference being that its salts are less lipophilic and more easily crystallized.

An ionic liquid (IL) is a salt in the liquid state. In some contexts, the term has been restricted to salts whose melting point is below some arbitrary temperature, such as 100 °C (212 °F). While ordinary liquids such as water and gasoline are predominantly made of electrically neutral molecules, ionic liquids are largely made of ions. These substances are variously called liquid electrolytes, ionic melts, ionic fluids, fused salts, liquid salts, or ionic glasses.

Ion exchange is a reversible interchange of one kind of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid with the reaction being used especially for softening or making water demineralised, the purification of chemicals and separation of substances.
In chemistry, the phosphonium cation describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Anions that interact weakly with cations are termed non-coordinating anions, although a more accurate term is weakly coordinating anion. Non-coordinating anions are useful in studying the reactivity of electrophilic cations. They are commonly found as counterions for cationic metal complexes with an unsaturated coordination sphere. These special anions are essential components of homogeneous alkene polymerisation catalysts, where the active catalyst is a coordinatively unsaturated, cationic transition metal complex. For example, they are employed as counterions for the 14 valence electron cations [(C5H5)2ZrR]+ (R = methyl or a growing polyethylene chain). Complexes derived from non-coordinating anions have been used to catalyze hydrogenation, hydrosilylation, oligomerization, and the living polymerization of alkenes. The popularization of non-coordinating anions has contributed to increased understanding of agostic complexes wherein hydrocarbons and hydrogen serve as ligands. Non-coordinating anions are important components of many superacids, which result from the combination of Brønsted acids and Lewis acids.

In organic chemistry, a radical anion is a free radical species that carries a negative charge. Radical anions are encountered in organic chemistry as reduced derivatives of polycyclic aromatic compounds, e.g. sodium naphthenide. An example of a non-carbon radical anion is the superoxide anion, formed by transfer of one electron to an oxygen molecule. Radical anions are typically indicated by .
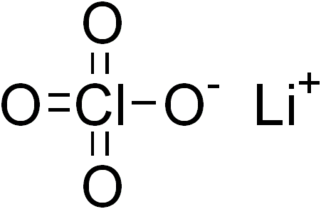
Lithium perchlorate is the inorganic compound with the formula LiClO4. This white or colourless crystalline salt is noteworthy for its high solubility in many solvents. It exists both in anhydrous form and as a trihydrate.
A salt metathesis reaction, sometimes called a double displacement reaction, is a chemical process involving the exchange of bonds between two reacting chemical species which results in the creation of products with similar or identical bonding affiliations. This reaction is represented by the general scheme:
In chemistry, a phase-transfer catalyst or PTC is a catalyst that facilitates the transition of a reactant from one phase into another phase where reaction occurs. Phase-transfer catalysis is a special form of heterogeneous catalysis. Ionic reactants are often soluble in an aqueous phase but insoluble in an organic phase in the absence of the phase-transfer catalyst. The catalyst functions like a detergent for solubilizing the salts into the organic phase. Phase-transfer catalysis refers to the acceleration of the reaction upon the addition of the phase-transfer catalyst.

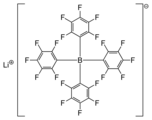
Tetrafluoroborate is the anion BF−
4. This tetrahedral species is isoelectronic with tetrafluoroberyllate (BeF2−
4), tetrafluoromethane (CF4), and tetrafluoroammonium (NF+
4) and is valence isoelectronic with many stable and important species including the perchlorate anion, ClO−
4, which is used in similar ways in the laboratory. It arises by the reaction of fluoride salts with the Lewis acid BF3, treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid.

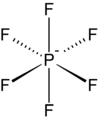
Hexafluorophosphate is an anion with chemical formula of [PF6]−. It is an octahedral species that imparts no color to its salts. [PF6]− is isoelectronic with sulfur hexafluoride, SF6, and the hexafluorosilicate dianion, [SiF6]2−, and hexafluoroantimonate [SbF6]−. In this anion, phosphorus has a valence of 5. Being poorly nucleophilic, hexafluorophosphate is classified as a non-coordinating anion.
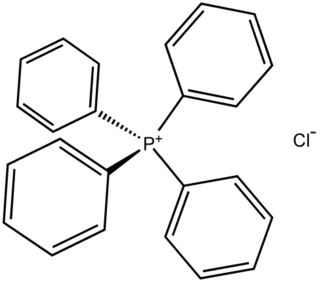
Tetraphenylphosphonium chloride is the chemical compound with the formula (C6H5)4PCl, abbreviated Ph4PCl or PPh4Cl. Tetraphenylphosphonium and especially tetraphenylarsonium salts were formerly of interest in gravimetric analysis of perchlorate and related oxyanions. This colourless salt is used to generate lipophilic salts from inorganic and organometallic anions. Thus, Ph4P+ is useful as a phase-transfer catalyst, again because it allows inorganic anions to dissolve in organic solvents.
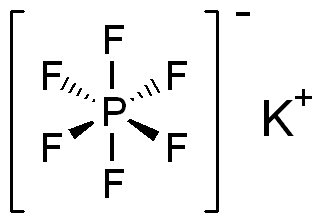
Potassium hexafluorophosphate is the chemical compound with the formula KPF6. This colourless salt consists of potassium cations and hexafluorophosphate anions. It is prepared by the reaction:
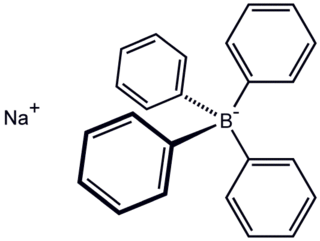
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron. This white crystalline solid is used to prepare other tetraphenylborate salts, which are often highly soluble in organic solvents. The compound is used in inorganic and organometallic chemistry as a precipitating agent for potassium, ammonium, rubidium, and cesium ions, and some organic nitrogen compounds.

Tetrakis(acetonitrile)copper(I) hexafluorophosphate is a salt with the formula [Cu(CH3CN)4]PF6. It is a colourless solid that is used in the synthesis of other copper complexes. The cation [Cu(CH3CN)4]+ is a well-known example of a transition metal nitrile complex.

Ferrocenium tetrafluoroborate is an organometallic compound with the formula [Fe(C5H5)2]BF4. This salt is composed of the cation [Fe(C5H5)2]+ and the tetrafluoroborate anion (BF−
4). The related hexafluorophosphate is also a popular reagent with similar properties. The cation is often abbreviated Fc+ or Cp2Fe+. The salt is deep blue in color and paramagnetic. Ferrocenium salts are sometimes used as one-electron oxidizing agents, and the reduced product, ferrocene, is inert and readily separated from ionic products. The ferrocene–ferrocenium couple is often used as a reference in electrochemistry. The standard potential of ferrocene-ferrocenium is 0.400 V vs. the normal hydrogen electrode (NHE) and is often assumed to be invariant between different solvents.

Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]−, indicating the presence of fluorinated aryl (ArF) groups. It is sometimes referred to as Kobayashi's anion in honour of Hiroshi Kobayashi who led the team that first synthesised it. More commonly it is affectionately nicknamed "BARF." The BARF ion is also abbreviated BArF24−, to distinguish it from the closely related BArF−
20, [(C6F5)4B]−.
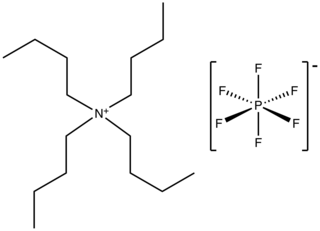
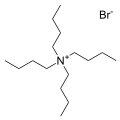
Tetrabutylammonium hexafluorophosphate is a salt with the formula NBu4PF6. It is a white powder that is used as an electrolyte in nonaqueous electrochemistry. It is highly soluble in polar organic solvents such as acetone and acetonitrile.