In organic chemistry, the Diels–Alder reaction is a chemical reaction between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted mechanism. More specifically, it is classified as a thermally-allowed [4+2] cycloaddition with Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in the synthesis of natural products and new materials. The underlying concept has also been applied to π-systems involving heteroatoms, such as carbonyls and imines, which furnish the corresponding heterocycles; this variant is known as the hetero-Diels–Alder reaction. The reaction has also been generalized to other ring sizes, although none of these generalizations have matched the formation of six-membered rings in terms of scope or versatility. Because of the negative values of ΔH° and ΔS° for a typical Diels–Alder reaction, the microscopic reverse of a Diels–Alder reactions becomes favorable at high temperatures, although this is of synthetic importance for only a limited range of Diels-Alder adducts, generally with some special structural features; this reverse reaction is known as the retro-Diels–Alder reaction.
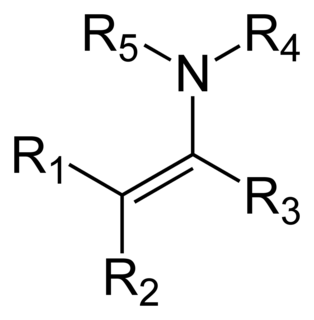
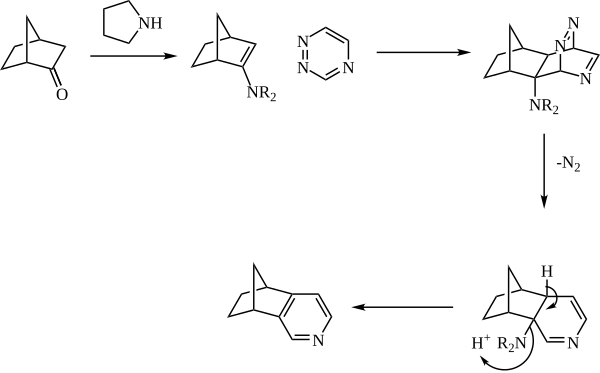
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.

An imine is a functional group or chemical compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen (H) or an organic group (R). If this group is not a hydrogen atom, then the compound can sometimes be referred to as a Schiff base. The carbon atom has two additional single bonds. The term "imine" was coined in 1883 by the German chemist Albert Ladenburg.
Thiazole, or 1,3-thiazole, is a heterocyclic compound that contains both sulfur and nitrogen; the term 'thiazole' also refers to a large family of derivatives. Thiazole itself is a pale yellow liquid with a pyridine-like odor and the molecular formula C3H3NS. The thiazole ring is notable as a component of the vitamin thiamine (B1).
An alkyne trimerisation reaction is a 2+2+2 cyclization reaction in which three molecules of alkyne react to form an arene. The reaction requires metal catalysts. The process is of historic interest as well as being applicable to organic synthesis. Many variations have been developed including cyclization of mixtures of alkynes and alkenes as well as alkynes and nitriles.
A dendralene is a discrete acyclic cross-conjugated polyene. The simplest dendralene is buta-1,3-diene (1) or [2]dendralene followed by [3]dendralene (2), [4]dendralene (3) and [5]dendralene (4) and so forth. [2]dendralene (butadiene) is the only one not cross-conjugated.

The Povarov reaction is an organic reaction described as a formal cycloaddition between an aromatic imine and an alkene. The imine in this organic reaction is a condensation reaction product from an aniline type compound and a benzaldehyde type compound. The alkene must be electron rich which means that functional groups attached to the alkene must be able to donate electrons. Such alkenes are enol ethers and enamines. The reaction product in the original Povarov reaction is a quinoline. Because the reactions can be carried out with the three components premixed in one reactor it is an example of a multi-component reaction.
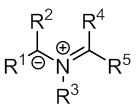
Azomethine ylides are nitrogen-based 1,3-dipoles, consisting of an iminium ion next to a carbanion. They are used in 1,3-dipolar cycloaddition reactions to form 5-membered heterocycles, including pyrrolidines and pyrrolines. These reactions are highly stereo- and regioselective, and have the potential to form four new contiguous stereocenters. Azomethine ylides thus have high utility in total synthesis, and formation of chiral ligands and pharmaceuticals. Azomethine ylides can be generated from many sources, including aziridines, imines, and iminiums. They are often generated in situ, and immediately reacted with dipolarophiles.
Endohedral hydrogen fullerene (H2@C60) is an endohedral fullerene containing molecular hydrogen. This chemical compound has a potential application in molecular electronics and was synthesized in 2005 at Kyoto University by the group of Koichi Komatsu. Ordinarily the payload of endohedral fullerenes are inserted at the time of the synthesis of the fullerene itself or is introduced to the fullerene at very low yields at high temperatures and high pressure. This particular fullerene was synthesised in an unusual way in three steps starting from pristine C60 fullerene: cracking open the carbon framework, insert hydrogen gas and zipping up by organic synthesis methods.

Danishefsky’s diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-1,3-butadiene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.
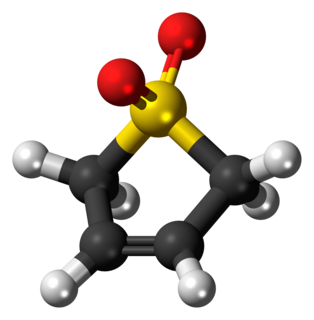
Sulfolene, or butadiene sulfone is a cyclic organic chemical with a sulfone functional group. It is a white, odorless, crystalline, indefinitely storable solid, which dissolves in water and many organic solvents. The occasionally reported pungent smell probably originates from adhering sulfur dioxide. The compound is used as a source of butadiene, but it is a versatile synthetic intermediate.In the laboratory it is used as a solid source of butadiene, into which it decomposes by a reverse cycloaddition.
In organic chemistry, an intramolecular Diels-Alder cycloaddition is a Diels–Alder reaction in which the diene and a dienophile are both part of the same molecule. The reaction leads to the formation of the same cyclohexene-like structure as usual for a Diels–Alder reaction, but as part of a more complex fused or bridged cyclic ring system.

Sulfene is an extremely reactive chemical compound with the formula H2C=SO2. It is the simplest member of the sulfenes, the group of compounds which are S,S-dioxides of thioaldehydes and thioketones, and have the general formula R2C=SO2.
Arsabenzene (IUPAC name: arsinine) is an organoarsenic heterocyclic compound with the chemical formula C5H5As. It belongs to a group of compounds called heteroarenes that have the general formula C5H5E (E= N, P, As, Sb, Bi).
The imine Diels-Alder reaction involves the transformation of all-carbon dienes and imine dienophiles into tetrahydropyridines.

Torreyanic acid is a dimeric quinone first isolated and by Lee et al. in 1996 from an endophyte, Pestalotiopsis microspora. This endophyte is likely the cause of the decline of Florida torreya, an endangered species that is related to the taxol-producing Taxus brevifolia. The natural product was found to be cytotoxic against 25 different human cancer cell lines with an average IC50 value of 9.4 µg/mL, ranging from 3.5 (NEC) to 45 (A549) µg/mL. Torreyanic acid was found to be 5-10 times more potent in cell lines sensitive to protein kinase C (PKC) agonists, 12-o-tetradecanoyl phorbol-13-acetate (TPA), and was shown to cause cell death via apoptosis. Torreyanic acid also promoted G1 arrest of G0 cynchronized cells at 1-5 µg/mL levels, depending on the cell line. It has been proposed that the eukaryotic translation initiation factor EIF-4a is a potential biochemical target for the natural compound.
The Inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right.
Photoredox catalysis is a branch of catalysis that harnesses the energy of light to accelerate a chemical reaction via single-electron transfer events. This area is named as a combination of "photo-" referring to light and redox, a condensed expression for the chemical processes of reduction and oxidation. In particular, photoredox catalysis employs small quantities of a light-sensitive compound that, when excited by light, can mediate the transfer of electrons between chemical compounds that otherwise would react more slowly or not at all. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
A nitroalkene, or nitro olefin, is a functional group combining the functionality of its constituent parts, an alkene and nitro group, while displaying its own chemical properties through alkene activation, making the functional group useful in specialty reactions such as the Michael reaction or Diels-Alder additions.