The following outline is provided as an overview of and topical guide to organic chemistry:

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.

In organic chemistry, an imine is a functional group or organic compound containing a carbon–nitrogen double bond. The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions.
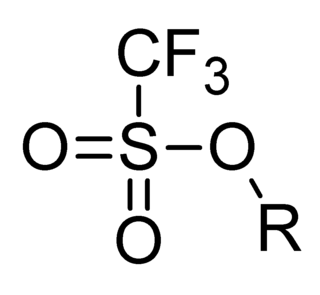
In organic chemistry, triflate, is a functional group with the formula R−OSO2CF3 and structure R−O−S(=O)2−CF3. The triflate group is often represented by −OTf, as opposed to −Tf, which is the triflyl group, R−SO2CF3. For example, n-butyl triflate can be written as CH3CH2CH2CH2OTf.
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more unsaturated molecules combine with the formation of a cyclic adduct in which there is a net reduction of the bond multiplicity". The resulting reaction is a cyclization reaction. Many but not all cycloadditions are concerted and thus pericyclic. Nonconcerted cycloadditions are not pericyclic. As a class of addition reaction, cycloadditions permit carbon–carbon bond formation without the use of a nucleophile or electrophile.
The 1,3-dipolar cycloaddition is a chemical reaction between a 1,3-dipole and a dipolarophile to form a five-membered ring. The earliest 1,3-dipolar cycloadditions were described in the late 19th century to the early 20th century, following the discovery of 1,3-dipoles. Mechanistic investigation and synthetic application were established in the 1960s, primarily through the work of Rolf Huisgen. Hence, the reaction is sometimes referred to as the Huisgen cycloaddition. 1,3-dipolar cycloaddition is an important route to the regio- and stereoselective synthesis of five-membered heterocycles and their ring-opened acyclic derivatives. The dipolarophile is typically an alkene or alkyne, but can be other pi systems. When the dipolarophile is an alkyne, aromatic rings are generally produced.

The Aza-Diels–Alder reaction is a modification of the Diels–Alder reaction wherein a nitrogen replaces sp2 carbon. The nitrogen atom can be part of the diene or the dienophile.

In stereochemistry, a chiral auxiliary is a stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxiliary can bias the stereoselectivity of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

The Doebner–Miller reaction is the organic reaction of an aniline with α,β-unsaturated carbonyl compounds to form quinolines.

Danishefsky's diene is an organosilicon compound and a diene with the formal name trans-1-methoxy-3-trimethylsilyloxy-buta-1,3-diene named after Samuel J. Danishefsky. Because the diene is very electron-rich it is a very reactive reagent in Diels-Alder reactions. This diene reacts rapidly with electrophilic alkenes, such as maleic anhydride. The methoxy group promotes highly regioselective additions. The diene is known to react with amines, aldehydes, alkenes and alkynes. Reactions with imines and nitro-olefins have been reported.
The Combes quinoline synthesis is a chemical reaction, which was first reported by Combes in 1888. Further studies and reviews of the Combes quinoline synthesis and its variations have been published by Alyamkina et al., Bergstrom and Franklin, Born, and Johnson and Mathews.

Benzylideneacetone is the organic compound described by the formula C6H5CH=CHC(O)CH3. Although both cis- and trans-isomers are possible for the α,β-unsaturated ketone, only the trans isomer is observed. Its original preparation demonstrated the scope of condensation reactions to construct new, complex organic compounds. Benzylideneacetone is used as a flavouring ingredient in food and perfumes.

The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids.
The Pfitzinger reaction is the chemical reaction of isatin with base and a carbonyl compound to yield substituted quinoline-4-carboxylic acids.
The inverse electron demand Diels–Alder reaction, or DAINV or IEDDA is an organic chemical reaction, in which two new chemical bonds and a six-membered ring are formed. It is related to the Diels–Alder reaction, but unlike the Diels–Alder reaction, the DAINV is a cycloaddition between an electron-rich dienophile and an electron-poor diene. During a DAINV reaction, three pi-bonds are broken, and two sigma bonds and one new pi-bond are formed. A prototypical DAINV reaction is shown on the right.
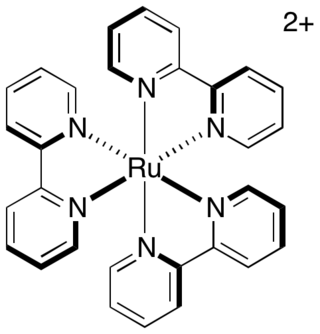
Photoredox catalysis is a branch of photochemistry that uses single-electron transfer. Photoredox catalysts are generally drawn from three classes of materials: transition-metal complexes, organic dyes, and semiconductors. While organic photoredox catalysts were dominant throughout the 1990s and early 2000s, soluble transition-metal complexes are more commonly used today.
In organic chemistry, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction. This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels–Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.
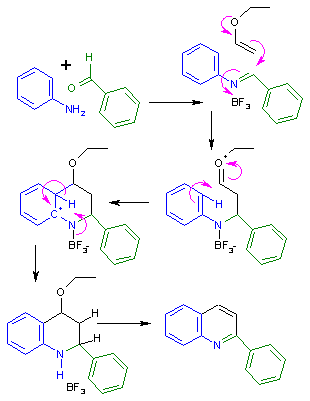
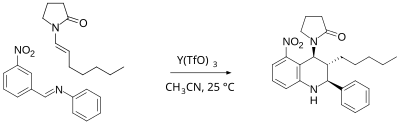
The Grieco three-component condensation is an organic chemistry reaction that produces nitrogen-containing six-member heterocycles via a multi-component reaction of an aldehyde, a nitrogen component, such as aniline, and an electron-rich alkene. The reaction is catalyzed by trifluoroacetic acid or Lewis acids such as ytterbium trifluoromethanesulfonate (Yb(OTf)3). The reaction is named for Paul Grieco, who first reported it in 1985. In the original paper the nitrogen component were benzylamine, methyl amine or ammonium chloride, the reaction now also include anilines, similar to the earlier Povarov reaction.

![Donor-acceptor cyclopropene formation and subsequent [4+2] cycloaddition to yield cyclopropane-fused tetrahydroquinolines. Subsequent treatment with TBAF opens the cyclopropane ring to give benzazepines. -4+2- cycloaddition of donor-acceptor cyclopropenes with aryl imines.png](http://upload.wikimedia.org/wikipedia/commons/thumb/4/43/-4%2B2-_cycloaddition_of_donor-acceptor_cyclopropenes_with_aryl_imines.png/500px--4%2B2-_cycloaddition_of_donor-acceptor_cyclopropenes_with_aryl_imines.png)











