The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids. [1] [2]
Contents

The reaction serves as an alternative to the Pfitzinger reaction. [3] [1]
The Doebner reaction is the chemical reaction of an aniline with an aldehyde and pyruvic acid to form quinoline-4-carboxylic acids. [1] [2]

The reaction serves as an alternative to the Pfitzinger reaction. [3] [1]
The reaction mechanism is not exactly known; two proposals are presented here. One possibility is at first an aldol condensation, starting from the enol form of the pyruvic acid (1) and the aldehyde, forming an β,γ-unsaturated α-ketocarboxylic acid (2). This is followed by a Michael addition with aniline to form an aniline derivative (3). After a cyclization at the benzene ring and two proton shifts, the quinoline-4-carboxylic acid (4) is formed by water elimination: [4]

An alternative mechanism is based on the aniline and the aldehyde forming at first the Schiff base upon water elimination. The subsequent reaction with the enol form of pyruvic acid (1) leads to the formation of the above-mentioned aniline derivative (3) followed by the above-described reaction mechanism: [4]

It is reported in the literature that the Doebner reaction fails in case of 2-chloro-5-aminopyridine. In this case the cyclization would take place at the amino group instead of the benzene ring and lead to a pyrrolidine derivative. [5]
Alternative syntheses of quinoline derivatives are for example: [4] [3]
Pyrrole is a heterocyclic, aromatic, organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

Quinoline is a heterocyclic aromatic organic compound with the chemical formula C9H7N. It is a colorless hygroscopic liquid with a strong odor. Aged samples, especially if exposed to light, become yellow and later brown. Quinoline is only slightly soluble in cold water but dissolves readily in hot water and most organic solvents. Quinoline itself has few applications, but many of its derivatives are useful in diverse applications. A prominent example is quinine, an alkaloid found in plants. Over 200 biologically active quinoline and quinazoline alkaloids are identified. 4-Hydroxy-2-alkylquinolines (HAQs) are involved in antibiotic resistance.

An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.
The Friedländer synthesis is a chemical reaction of 2-aminobenzaldehydes with ketones to form quinoline derivatives. It is named after German chemist Paul Friedländer (1857–1923).
The Niementowski quinoline synthesis is the chemical reaction of anthranilic acids and ketones to form γ-hydroxyquinoline derivatives.

The Petasis reaction is the multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines.
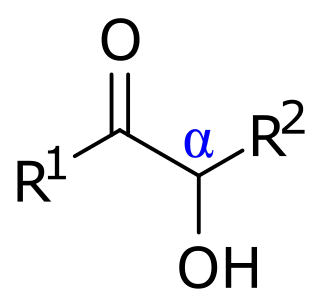
In organic chemistry, acyloins or α-hydroxy ketones are a class of organic compounds of the general form R−C(=O)−CR'(OH)−R", composed of a hydroxy group adjacent to a ketone group. The name acyloin is derived from the fact that they are formally derived from reductive coupling of carboxylic acyl groups. They are one of the two main classes of hydroxy ketones, distinguished by the position of the hydroxy group relative to the ketone; in this form, the hydroxy is on the alpha carbon, explaining the secondary name of α-hydroxy ketone.

The Doebner–Miller reaction is the organic reaction of an aniline with α,β-unsaturated carbonyl compounds to form quinolines.

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.
The Conrad–Limpach synthesis is the condensation of anilines (1) with β-ketoesters (2) to form 4-hydroxyquinolines (4) via a Schiff base (3). The overall reaction type is a combination of both an addition reaction as well as a rearrangement reaction. This reaction was discovered by Max Conrad (1848–1920) and Leonhard Limpach (1852–1933) in 1887 while they were studying the synthesis of quinoline derivatives.
The Rubottom oxidation is a useful, high-yielding chemical reaction between silyl enol ethers and peroxyacids to give the corresponding α-hydroxy carbonyl product. The mechanism of the reaction was proposed in its original disclosure by A.G. Brook with further evidence later supplied by George M. Rubottom. After a Prilezhaev-type oxidation of the silyl enol ether with the peroxyacid to form the siloxy oxirane intermediate, acid-catalyzed ring-opening yields an oxocarbenium ion. This intermediate then participates in a 1,4-silyl migration to give an α-siloxy carbonyl derivative that can be readily converted to the α-hydroxy carbonyl compound in the presence of acid, base, or a fluoride source.
The Combes quinoline synthesis is a chemical reaction, which was first reported by Combes in 1888. Further studies and reviews of the Combes quinoline synthesis and its variations have been published by Alyamkina et al., Bergstrom and Franklin, Born, and Johnson and Mathews.
In organic chemistry, the Baudisch reaction is a process for the synthesis of nitrosophenols using metal ions. Although the products are of limited value, the reaction is of historical interest as an example of metal-promoted functionalization of aromatic substrates.
In organic chemistry, a homologation reaction, also known as homologization, is any chemical reaction that converts the reactant into the next member of the homologous series. A homologous series is a group of compounds that differ by a constant unit, generally a methylene group. The reactants undergo a homologation when the number of a repeated structural unit in the molecules is increased. The most common homologation reactions increase the number of methylene units in saturated chain within the molecule. For example, the reaction of aldehydes or ketones with diazomethane or methoxymethylenetriphenylphosphine to give the next homologue in the series.
The Pfitzinger reaction is the chemical reaction of isatin with base and a carbonyl compound to yield substituted quinoline-4-carboxylic acids.

The Gould–Jacobs reaction is an organic synthesis for the preparation of quinolines and 4‐hydroxyquinoline derivatives. The Gould–Jacobs reaction is a series of reactions. The series of reactions begins with the condensation/substitution of an aniline with alkoxy methylenemalonic ester or acyl malonic ester, producing anilidomethylenemalonic ester. Then through a 6 electron cyclization process, 4-hydroxy-3-carboalkoxyquinoline is formed, which exist mostly in the 4-oxo form. Saponification results in the formation of an acid. This step is followed by decarboxylation to give 4-hydroxyquinoline. The Gould–Jacobs reaction is effective for anilines with electron‐donating groups at the meta‐position.
The Ferrier carbocyclization is an organic reaction that was first reported by the carbohydrate chemist Robert J. Ferrier in 1979. It is a metal-mediated rearrangement of enol ether pyrans to cyclohexanones. Typically, this reaction is catalyzed by mercury salts, specifically mercury(II) chloride.

Indole is an organic compound with the formula C6H4CCNH3. Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole where one or more of the hydrogen atoms have been replaced by substituent groups. Indoles are widely distributed in nature, most notably as amino acid tryptophan and neurotransmitter serotonin.

4,7-Dichloroquinoline is a two-ring heterocyclic compound used as a chemical intermediate to aminoquinoline antimalarial drugs including amodiaquine, chloroquine and hydroxychloroquine.