| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Pyrrolidine [1] | |||
| Other names Azolidine Azacyclopentane Tetrahydropyrrole Prolamine Azolane | |||
| Identifiers | |||
3D model (JSmol) | |||
| 102395 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.004.227 | ||
| EC Number |
| ||
| 1704 | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1922 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
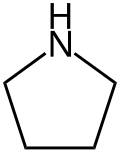
| C4H9N | |||
| Molar mass | 71.123 g·mol−1 | ||
| Appearance | Clear colorless liquid | ||
| Density | 0.866 g/cm3 | ||
| Melting point | −63 °C (−81 °F; 210 K) | ||
| Boiling point | 87 °C (189 °F; 360 K) | ||
| Miscible | |||
| Acidity (pKa) | 11.27 (pKa of conjugate acid in water), [2] 19.56 (pKa of conjugate acid in acetonitrile) [3] | ||
| −54.8·10−6 cm3/mol | |||
Refractive index (nD) | 1.4402 at 28°C | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | highly flammable, harmful, corrosive, possible mutagen | ||
| GHS labelling: | |||
   | |||
| Danger | |||
| H225, H302, H314, H332 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P301+P312, P301+P330+P331, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P330, P363, P370+P378, P403+P235, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 3 °C (37 °F; 276 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Safety data sheet (SDS) | MSDS | ||
| Related compounds | |||
Related nitrogen heterocyclic compounds | Pyrrole (aromatic with two double bonds) Pyrroline (one double bond) Pyrrolizidine (two pentagonal rings) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Pyrrolidine, also known as tetrahydropyrrole, is an organic compound with the molecular formula (CH2)4NH. It is a cyclic secondary amine, also classified as a saturated heterocycle. It is a colourless liquid that is miscible with water and most organic solvents. It has a characteristic odor that has been described as "ammoniacal, fishy, shellfish-like". [4] In addition to pyrrolidine itself, many substituted pyrrolidines are known.