Non-collagen
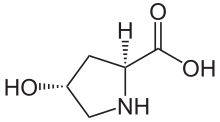
Hydroxyproline is found in few proteins other than collagen. For this reason, hydroxyproline content has been used as an indicator to determine collagen and/or gelatin amount. However, the mammalian proteins elastin and argonaute 2 have collagen-like domains in which hydroxyproline is formed. Some snail poisons, conotoxins, contain hydroxyproline, but lack collagen-like sequences. [2]
Hydroxylation of proline has been shown to be involved in targeting Hypoxia-inducible factor (HIF) alpha subunit (HIF-1 alpha) for degradation by proteolysis. Under normoxia (normal oxygen conditions) EGLN1 protein hydroxylates the proline at the 564 position of HIF-1 alpha, which allows ubiquitylation by the von Hippel-Lindau tumor suppressor (pVHL) and subsequent targeting for proteasome degradation. [8]
DYRK1A, DYRK1B, protein kinase B, eEF2, IKK2, p53, FOXO3A, CEP192 are also reportedly hydroxylated by PHD1. p53 and MAPH6 are also hydroxylated by PHD3. [9]
Free hydroxyproline appears to be an antioxidant, like free proline. [9]