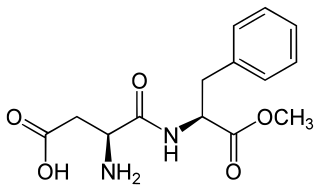
Aspartame is an artificial non-saccharide sweetener 200 times sweeter than sucrose and is commonly used as a sugar substitute in foods and beverages. It is a methyl ester of the aspartic acid/phenylalanine dipeptide with brand names NutraSweet, Equal, and Canderel. Aspartame was approved by the US Food and Drug Administration (FDA) in 1974, and then again in 1981, after approval was revoked in 1980.

Biopolymers are natural polymers produced by the cells of living organisms. Like other polymers, biopolymers consist of monomeric units that are covalently bonded in chains to form larger molecules. There are three main classes of biopolymers, classified according to the monomers used and the structure of the biopolymer formed: polynucleotides, polypeptides, and polysaccharides. The Polynucleotides, RNA and DNA, are long polymers of nucleotides. Polypeptides include proteins and shorter polymers of amino acids; some major examples include collagen, actin, and fibrin. Polysaccharides are linear or branched chains of sugar carbohydrates; examples include starch, cellulose, and alginate. Other examples of biopolymers include natural rubbers, suberin and lignin, cutin and cutan, melanin, and polyhydroxyalkanoates (PHAs).

Gelatin desserts are desserts made with a sweetened and flavoured processed collagen product (gelatin). This kind of dessert was first recorded as jelly by Hannah Glasse in her 18th-century book The Art of Cookery, appearing in a layer of trifle. Jelly is also featured in the best selling cookbooks of English food writers Eliza Acton and Isabella Beeton in the 19th century.

Carrageenans or carrageenins are a family of natural linear sulfated polysaccharides that are extracted from red edible seaweeds. Carrageenans are widely used in the food industry, for their gelling, thickening, and stabilizing properties. Their main application is in dairy and meat products, due to their strong binding to food proteins. In recent years, carrageenans have emerged as a promising candidate in tissue engineering and regenerative medicine applications as they resemble native glycosaminoglycans (GAGs). They have been mainly used for tissue engineering, wound coverage, and drug delivery.

Gummy bears are small, fruit gum candies, similar to a jelly baby in some English-speaking countries. The candy is roughly 2 cm (0.8 in) long and shaped in the form of a bear. The gummy bear is one of many gummies, popular gelatin-based candies sold in a variety of shapes and colors.

Mechanically separated meat (MSM), mechanically recovered/reclaimed meat (MRM), or mechanically deboned meat (MDM) is a paste-like meat product produced by forcing pureed or ground beef, pork, mutton, turkey or chicken under high pressure through a sieve or similar device to separate the bone from the edible meat tissue. When poultry is used, it is sometimes called white slime as an analog to meat-additive pink slime and to meat extracted by advanced meat recovery systems, both of which are different processes. The process entails pureeing or grinding the carcass left after the manual removal of meat from the bones and then forcing the slurry through a sieve under pressure.
Calorad Classic is a liquid protein weight loss supplement which was first introduced to the US and Canadian marketplace in 1984. It has been advertised on both television and radio. Calorad Classic is manufactured by NutriDiem and is marketed by several companies including Essentially Yours Industries and Nysante, all of which are headquartered in Canada.

Whey protein is a mixture of proteins isolated from whey, the liquid material created as a by-product of cheese production. The proteins consist of α-lactalbumin, β-lactoglobulin, serum albumin and immunoglobulins. Glycomacropeptide also makes up the third largest component but is not a protein. Whey protein is commonly marketed as a protein supplement, and various health claims have been attributed to it. A review published in 2010 in the European Food Safety Authority Journal concluded that the provided literature did not adequately support the proposed claims.
Hydrolyzed protein is a solution derived from the hydrolysis of a protein into its component amino acids and peptides. While many means of achieving this exist, most common is prolonged heating with hydrochloric acid, sometimes with an enzyme such as pancreatic protease to simulate the naturally occurring hydrolytic process.
Hydrolyzed vegetable protein (HVP) products are foodstuffs obtained by protein hydrolysis and are used as ingredients to create a bouillon (broth) taste without the vegetables, bones, simmering, or other standard elements of creating bouillon from scratch.

A thickening agent or thickener is a substance which can increase the viscosity of a liquid without substantially changing its other properties. Edible thickeners are commonly used to thicken sauces, soups, and puddings without altering their taste; thickeners are also used in paints, inks, explosives, and cosmetics.

Caramel color or caramel coloring is a water-soluble food coloring. It is made by heat treatment of carbohydrates (sugars), in general in the presence of acids, alkalis, or salts, in a process called caramelization. It is more fully oxidized than caramel candy, and has an odor of burnt sugar and a somewhat bitter taste. Its color ranges from pale yellow to amber to dark brown.
John Mark Purdey was an English organic farmer who came to public attention in the 1980s, when he began to circulate his own theories regarding the causes of bovine spongiform encephalopathy.

Bovine spongiform encephalopathy (BSE), commonly known as mad cow disease, is an incurable and invariably fatal neurodegenerative disease of cattle. Symptoms include abnormal behavior, trouble walking, and weight loss. Later in the course of the disease, the cow becomes unable to function normally. There is conflicting information about the time between infection and onset of symptoms. In 2002, the World Health Organization (WHO) suggested it to be approximately four to five years. Time from onset of symptoms to death is generally weeks to months. Spread to humans is believed to result in variant Creutzfeldt–Jakob disease (vCJD). As of 2018, a total of 231 cases of vCJD had been reported globally.

Meat and bone meal (MBM) is a product of the rendering industry. It is typically about 48–52% protein, 33–35% ash, 8–12% fat, and 4–7% water. It is primarily used in the formulation of animal feed to improve the amino acid profile of the feed. Feeding of MBM to cattle is thought to have been responsible for the spread of BSE ; therefore, in most parts of the world, MBM is no longer allowed in feed for ruminant animals. However, it is still used to feed monogastric animals.
The term Feed ban is usually a reference to the regulations that have prohibited the feeding of most mammalian-derived proteins to cattle as a method of preventing the spread of bovine spongiform encephalopathy (BSE). Feeding of infected ruminant material back to ruminants is believed to be the most likely means of transmission of the disease.

Meat on the bone or bone-in meat is meat that is sold with some or all of the bones included in the cut or portion, i.e. meat that has not been filleted. The phrase "on the bone" can also be applied to specific types of meat, most commonly ham on the bone, and to fish. Meat or fish on the bone may be cooked and served with the bones still included or the bones may be removed at some stage in the preparation.
Ossein is the organic extracellular matrix of bone, which is made of 95% collagen. This substance is used in industry for the production of gelatin and bone glue.

The United Kingdom was afflicted with an outbreak of Bovine spongiform encephalopathy, and its human equivalent variant Creutzfeldt–Jakob disease (vCJD), in the 1980s and 1990s. Over four million head of cattle were slaughtered in an effort to contain the outbreak, and 178 people died after contracting vCJD through eating infected beef. A political and public health crisis resulted, and British beef was banned from export to numerous countries around the world, with some bans remaining in place until as late as 2019.

The Beef Bones Regulations 1997 was a statutory instrument of the British government that limited the sale of beef on the bone. The regulations were implemented as a response to the United Kingdom BSE outbreak over fears that variant Creutzfeldt–Jakob disease in humans might be caused by the consumption of dorsal root ganglia, which lie near the bone. As well as beef on the bone, all beef-bone derived products were prohibited from sale. This had the effect of outlawing T-bone steaks, prime ribs and oxtail as well as some soups and stocks. Other aspects of the regulations dealt with the deboning of beef and the keeping of records in the food production industry. The restrictions on sales were lifted in December 1999 and the regulations as a whole were revoked in April 2008.