
Integrins are transmembrane receptors that help cell-cell and cell-extracellular matrix (ECM) adhesion. Upon ligand binding, integrins activate signal transduction pathways that mediate cellular signals such as regulation of the cell cycle, organization of the intracellular cytoskeleton, and movement of new receptors to the cell membrane. The presence of integrins allows rapid and flexible responses to events at the cell surface.

A pseudopod or pseudopodium is a temporary arm-like projection of a eukaryotic cell membrane that is emerged in the direction of movement. Filled with cytoplasm, pseudopodia primarily consist of actin filaments and may also contain microtubules and intermediate filaments. Pseudopods are used for motility and ingestion. They are often found in amoebas.

In biology, the extracellular matrix (ECM), also called intercellular matrix (ICM), is a network consisting of extracellular macromolecules and minerals, such as collagen, enzymes, glycoproteins and hydroxyapatite that provide structural and biochemical support to surrounding cells. Because multicellularity evolved independently in different multicellular lineages, the composition of ECM varies between multicellular structures; however, cell adhesion, cell-to-cell communication and differentiation are common functions of the ECM.

In cell biology, focal adhesions are large macromolecular assemblies through which mechanical force and regulatory signals are transmitted between the extracellular matrix (ECM) and an interacting cell. More precisely, focal adhesions are the sub-cellular structures that mediate the regulatory effects of a cell in response to ECM adhesion.

Podosomes are conical, actin-rich structures found on the outer surface of the plasma membrane of animal cells. Their size ranges from approximately 0.5 μm to 2.0 μm in diameter. While usually situated on the periphery of the cellular membrane, these unique structures display a polarized pattern of distribution in migrating cells, situating at the front border between the lamellipodium and lamellum. Their primary purpose is connected to cellular motility and invasion; therefore, they serve as both sites of attachment and degradation along the extracellular matrix. Many different specialized cells exhibit these dynamic structures such as invasive cancer cells, osteoclasts, vascular smooth muscle cells, endothelial cells, and certain immune cells like macrophages and dendritic cells.
Intravasation is the invasion of cancer cells through the basement membrane into a blood or lymphatic vessel. Intravasation is one of several carcinogenic events that initiate the escape of cancerous cells from their primary sites. Other mechanisms include invasion through basement membranes, extravasation, and colonization of distant metastatic sites. Cancer cell chemotaxis also relies on this migratory behavior to arrive at a secondary destination designated for cancer cell colonization.

Cortactin is a monomeric protein located in the cytoplasm of cells that can be activated by external stimuli to promote polymerization and rearrangement of the actin cytoskeleton, especially the actin cortex around the cellular periphery. It is present in all cell types. When activated, it will recruit Arp2/3 complex proteins to existing actin microfilaments, facilitating and stabilizing nucleation sites for actin branching. Cortactin is important in promoting lamellipodia formation, invadopodia formation, cell migration, and endocytosis.
Fascin is an actin bundling protein.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.

PTK2 protein tyrosine kinase 2 (PTK2), also known as focal adhesion kinase (FAK), is a protein that, in humans, is encoded by the PTK2 gene. PTK2 is a focal adhesion-associated protein kinase involved in cellular adhesion and spreading processes. It has been shown that when FAK was blocked, breast cancer cells became less metastatic due to decreased mobility.

ROCK1 is a protein serine/threonine kinase also known as rho-associated, coiled-coil-containing protein kinase 1. Other common names are ROKβ and P160ROCK. ROCK1 is a major downstream effector of the small GTPase RhoA and is a regulator of the actomyosin cytoskeleton which promotes contractile force generation. ROCK1 plays a role in cancer and in particular cell motility, metastasis, and angiogenesis.

Breast cancer anti-estrogen resistance protein 1 is a protein that in humans is encoded by the BCAR1 gene.

RhoC is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RHOC.
The Akt signaling pathway or PI3K-Akt signaling pathway is a signal transduction pathway that promotes survival and growth in response to extracellular signals. Key proteins involved are PI3K and Akt.

In medicine, desmoplasia is the growth of fibrous connective tissue. It is also called a desmoplastic reaction to emphasize that it is secondary to an insult. Desmoplasia may occur around a neoplasm, causing dense fibrosis around the tumor, or scar tissue (adhesions) within the abdomen after abdominal surgery.
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.
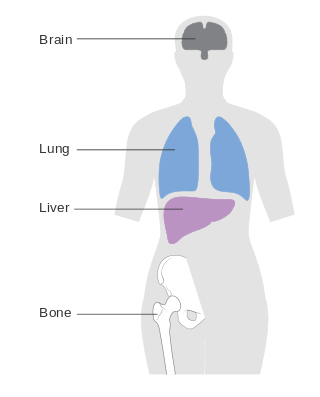
Metastatic breast cancer, also referred to as metastases, advanced breast cancer, secondary tumors, secondaries or stage IV breast cancer, is a stage of breast cancer where the breast cancer cells have spread to distant sites beyond the axillary lymph nodes. There is no cure for metastatic breast cancer; there is no stage after IV.

Cellular adhesions can be defined as proteins or protein aggregates that form mechanical and chemical linkages between the intracellular and extracellular space. Adhesions serve several critical processes including cell migration, signal transduction, tissue development and repair. Due to this functionality, adhesions and adhesion molecules have been a topic of study within the scientific community. Specifically, it has been found that adhesions are involved in tissue development, plasticity, and memory formation within the central nervous system (CNS), and may prove vital in the generation of CNS-specific therapeutics.
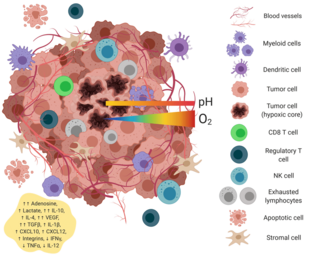
The tumor microenvironment is a complex ecosystem surrounding a tumor, composed of cancer cells, stromal tissue and the extracellular matrix. Mutual interaction between cancer cells and the different components of the tumor microenvironment support its growth and invasion in healthy tissues which correlates with tumor resistance to current treatments and poor prognosis. The tumor microenvironment is in constant change because of the tumor's ability to influence the microenvironment by releasing extracellular signals, promoting tumor angiogenesis and inducing peripheral immune tolerance, while the immune cells in the microenvironment can affect the growth and evolution of cancerous cells.

Invasion is the process by which cancer cells directly extend and penetrate into neighboring tissues in cancer. It is generally distinguished from metastasis, which is the spread of cancer cells through the circulatory system or the lymphatic system to more distant locations. Yet, lymphovascular invasion is generally the first step of metastasis.

















