Collagen, type II, alpha 1 (primary osteoarthritis, spondyloepiphyseal dysplasia, congenital), also known as COL2A1, is a human gene that provides instructions for the production of the pro-alpha1(II) chain of type II collagen.
Collagen, type II, alpha 1 (primary osteoarthritis, spondyloepiphyseal dysplasia, congenital), also known as COL2A1, is a human gene that provides instructions for the production of the pro-alpha1(II) chain of type II collagen.
This gene encodes the alpha-1 chain of type II collagen, a fibrillar collagen found in cartilage and the vitreous humor of the eye. Mutations in this gene are associated with achondrogenesis, chondrodysplasia, early onset familial osteoarthritis, SED congenita, Langer-Saldino achondrogenesis, Kniest dysplasia, Stickler syndrome type I, and spondyloepimetaphyseal dysplasia Strudwick type. In addition, defects in processing chondrocalcin, a calcium binding protein that is the C-propeptide of this collagen molecule, are also associated with chondrodysplasia. There are two transcripts identified for this gene. [5] Type II collagen, which adds structure and strength to connective tissues, is found primarily in cartilage, the jelly-like substance that fills the eyeball (the vitreous), the inner ear, and the center portion of the discs between the vertebrae in the spine (nucleus pulposus). Three pro-alpha1(II) chains twist together to form a triple-stranded, ropelike procollagen molecule. These procollagen molecules must be processed by enzymes in the cell. Once these molecules are processed, they leave the cell and arrange themselves into long, thin fibrils that cross-link to one another in the spaces around cells. The cross-linkages result in the formation of very strong mature type II collagen fibers.
The COL2A1 gene is located on the long (q) arm of chromosome 12 between positions 13.11 and 13.2, from base pair 46,653,017 to base pair 46,684,527. The expression of COL2A1 is regulated by SOX-9 and retrotransposon gag-like-3 gene RTL3 in chondrocytes. [6]

Stickler syndrome is a group of rare genetic disorders affecting connective tissue, specifically collagen. Stickler syndrome is a subtype of collagenopathy, types II and XI. Stickler syndrome is characterized by distinctive facial abnormalities, ocular problems, hearing loss, and joint and skeletal problems. It was first studied and characterized by Gunnar B. Stickler in 1965.

Spondyloperipheral dysplasia is an autosomal dominant disorder of bone growth. The condition is characterized by flattened bones of the spine (platyspondyly) and unusually short fingers and toes (brachydactyly). Some affected individuals also have other skeletal abnormalities, short stature, nearsightedness (myopia), hearing loss, and mental retardation. Spondyloperipheral dysplasia is a subtype of collagenopathy, types II and XI.
The type II and XI collagenopathies are a group of disorders that affect connective tissue, the tissue that supports the body's joints and organs. These disorders are caused by defects in type II or type XI collagen. Collagens are complex molecules that provide structure, strength, and elasticity to connective tissue. Type II and type XI collagen disorders are grouped together because both types of collagen are components of the cartilage found in joints and the spinal column, the inner ear, and the jelly-like substance that fills the eyeball. The type II and XI collagenopathies result in similar clinical features.
Hypochondrogenesis is a severe genetic disorder causing malformations of bone growth. The condition is characterized by a short body and limbs and abnormal bone formation in the spine and pelvis.
Kniest dysplasia is a rare form of dwarfism caused by a mutation in the COL2A1 gene on chromosome 12. The COL2A1 gene is responsible for producing type II collagen. The mutation of COL2A1 gene leads to abnormal skeletal growth and problems with hearing and vision. What characterizes Kniest dysplasia from other type II osteochondrodysplasia is the level of severity and the dumb-bell shape of shortened long tubular bones.
Spondyloepiphyseal dysplasia congenita is a rare disorder of bone growth that results in dwarfism, characteristic skeletal abnormalities, and occasionally problems with vision and hearing. The name of the condition indicates that it affects the bones of the spine (spondylo-) and the ends of bones (epiphyses), and that it is present from birth (congenital). The signs and symptoms of spondyloepiphyseal dysplasia congenita are similar to, but milder than, the related skeletal disorders achondrogenesis type 2 and hypochondrogenesis. Spondyloepiphyseal dysplasia congenita is a subtype of collagenopathy, types II and XI.

Spondyloepimetaphyseal dysplasia, Strudwick type is an inherited disorder of bone growth that results in dwarfism, characteristic skeletal abnormalities, and problems with vision. The name of the condition indicates that it affects the bones of the spine (spondylo-) and two regions near the ends of bones. This type was named after the first reported patient with the disorder. Spondyloepimetaphyseal dysplasia, Strudwick type is a subtype of collagenopathy, types II and XI.

Chromosome 12 is one of the 23 pairs of chromosomes in humans. People normally have two copies of this chromosome. Chromosome 12 spans about 133 million base pairs and represents between 4 and 4.5 percent of the total DNA in cells.

Collagen, type I, alpha 1, also known as alpha-1 type I collagen, is a protein that in humans is encoded by the COL1A1 gene. COL1A1 encodes the major component of type I collagen, the fibrillar collagen found in most connective tissues, including cartilage.

Collagen alpha-2(XI) chain is a protein that in humans is encoded by the COL11A2 gene.

Platyspondylic lethal skeletal dysplasia, Torrance type is a severe disorder of bone growth. People with this condition have very short arms and legs, a small chest with short ribs, underdeveloped pelvic bones, and unusually short fingers and toes (brachydactyly). This disorder is also characterized by flattened spinal bones (platyspondyly) and abnormal curvature of the spine (lordosis).
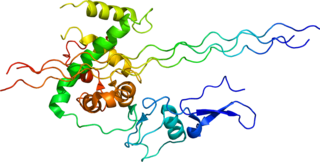
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the COL3A1 gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain.
Type I collagen is the most abundant collagen of the human body, consisting of around 90% of the body's total collagen in vertebrates. Due to this, it is also the most abundant protein type found in all vertebrates. Type I forms large, eosinophilic fibers known as collagen fibers, which make up most of the rope-like dense connective tissue in the body. Collagen I itself is created by the combination of both a proalpha1 and a proalpha2 chain created by the COL1alpha1 and COL1alpha2 genes respectively. The Col I gene itself takes up a triple-helical conformation due to its Glycine-X-Y structure, x and y being any type of amino acid. Collagen can also be found in two different isoforms, either as a homotrimer or a heterotrimer, both of which can be found during different periods of development. Heterotrimers, in particular, play an important role in wound healing, and are the dominant isoform found in the body.

The sulfate transporter is a solute carrier family protein that in humans is encoded by the SLC26A2 gene. SLC26A2 is also called the diastrophic dysplasia sulfate transporter (DTDST), and was first described by Hästbacka et al. in 1994. A defect in sulfate activation described by Superti-Furga in achondrogenesis type 1B was subsequently also found to be caused by genetic variants in the sulfate transporter gene. This sulfate (SO42−) transporter also accepts chloride, hydroxyl ions (OH−), and oxalate as substrates. SLC26A2 is expressed at high levels in developing and mature cartilage, as well as being expressed in lung, placenta, colon, kidney, pancreas and testis.

Cartilage oligomeric matrix protein (COMP), also known as thrombospondin-5, is an extracellular matrix (ECM) protein primarily present in cartilage. In humans it is encoded by the COMP gene.

Collagen alpha-1(IX) chain is a protein that in humans is encoded by the COL9A1 gene.

Collagen alpha-1(XI) chain is a protein that in humans is encoded by the COL11A1 gene.

Collagen alpha-2(IX) chain is a protein that in humans is encoded by the COL9A2 gene.
Cranio-lenticulo-sutural dysplasia is a neonatal/infancy disease caused by a disorder in the 14th chromosome. It is an autosomal recessive disorder, meaning that both recessive genes must be inherited from each parent in order for the disease to manifest itself. The disease causes a significant dilation of the endoplasmic reticulum in fibroblasts of the host with CLSD. Due to the distension of the endoplasmic reticulum, export of proteins from the cell is disrupted.

Spondylo-meta-epiphyseal dysplasia (SMED) is a rare autosomal-recessive disease that causes skeletal disorders. SMED is thought to be caused by a mutation in the Discoidin Domain Receptor 2 (DDR2) gene.