The Mumm rearrangement is an organic reaction and a rearrangement reaction. [1] [2] It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide.
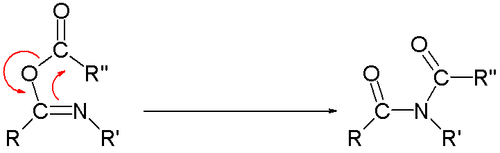
The reaction is of relevance as part of the Ugi reaction.
The Mumm rearrangement is an organic reaction and a rearrangement reaction. [1] [2] It describes a 1,3(O-N) acyl transfer of an acyl imidate or isoimide group to an imide.

The reaction is of relevance as part of the Ugi reaction.

In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula R−C(=O)−NR′R″, where R, R', and R″ represent any group, typically organyl groups or hydrogen atoms. The amide group is called a peptide bond when it is part of the main chain of a protein, and an isopeptide bond when it occurs in a side chain, as in asparagine and glutamine. It can be viewed as a derivative of a carboxylic acid with the hydroxyl group replaced by an amine group ; or, equivalently, an acyl (alkanoyl) group joined to an amine group.

In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group or hydrogen in the case of formyl group. In organic chemistry, the acyl group is usually derived from a carboxylic acid, in which case it has the formula R−C(=O)−, where R represents an organyl group or hydrogen. Although the term is almost always applied to organic compounds, acyl groups can in principle be derived from other types of acids such as sulfonic acids and phosphonic acids. In the most common arrangement, acyl groups are attached to a larger molecular fragment, in which case the carbon and oxygen atoms are linked by a double bond.
The following outline is provided as an overview of and topical guide to organic chemistry:

In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications, imides are best known as components of high-strength polymers, called polyimides. Inorganic imides are also known as solid state or gaseous compounds, and the imido group (=NH) can also act as a ligand.

Oxazole is the parent compound for a vast class of heterocyclic aromatic organic compounds. These are azoles with an oxygen and a nitrogen separated by one carbon. Oxazoles are aromatic compounds but less so than the thiazoles. Oxazole is a weak base; its conjugate acid has a pKa of 0.8, compared to 7 for imidazole.

An organic acid anhydride is an acid anhydride that is also an organic compound. An acid anhydride is a compound that has two acyl groups bonded to the same oxygen atom. A common type of organic acid anhydride is a carboxylic anhydride, where the parent acid is a carboxylic acid, the formula of the anhydride being (RC(O))2O. Symmetrical acid anhydrides of this type are named by replacing the word acid in the name of the parent carboxylic acid by the word anhydride. Thus, (CH3CO)2O is called acetic anhydride.Mixed (or unsymmetrical) acid anhydrides, such as acetic formic anhydride (see below), are known, whereby reaction occurs between two different carboxylic acids. Nomenclature of unsymmetrical acid anhydrides list the names of both of the reacted carboxylic acids before the word "anhydride" (for example, the dehydration reaction between benzoic acid and propanoic acid would yield "benzoic propanoic anhydride").
In organic chemistry, the Ugi reaction is a multi-component reaction involving a ketone or aldehyde, an amine, an isocyanide and a carboxylic acid to form a bis-amide. The reaction is named after Ivar Karl Ugi, who first reported this reaction in 1959.
The Pummerer rearrangement is an organic reaction whereby an alkyl sulfoxide rearranges to an α-acyloxy–thioether (monothioacetal-ester) in the presence of acetic anhydride.

The Claisen rearrangement is a powerful carbon–carbon bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl vinyl ether will initiate a [3,3]-sigmatropic rearrangement to give a γ,δ-unsaturated carbonyl, driven by exergonically favored carbonyl CO bond formation (Δ = −327 kcal/mol.

The Curtius rearrangement, first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a variety of nucleophiles such as water, alcohols and amines, to yield a primary amine, carbamate or urea derivative respectively. Several reviews have been published.
The Fries rearrangement, named for the German chemist Karl Theophil Fries, is a rearrangement reaction of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.
Hydrazides in organic chemistry are a class of organic compounds with the formula R−NR1−NR2R3 where R is acyl, sulfonyl, phosphoryl, phosphonyl and similar groups, R1, R2, R3 and R' are any groups. Unlike hydrazine and alkylhydrazines, hydrazides are nonbasic owing to the inductive influence of the acyl, sulfonyl, or phosphoryl substituent.

The Baker–Venkataraman rearrangement is the chemical reaction of 2-acetoxyacetophenones with base to form 1,3-diketones.
The Lossen rearrangement is the conversion of a hydroxamate ester to an isocyanate. Typically O-acyl, sulfonyl, or phosphoryl O-derivative are employed. The isocyanate can be used further to generate ureas in the presence of amines or generate amines in the presence of H2O.
The Gabriel–Colman rearrangement is the chemical reaction of a saccharin or phthalimido ester with a strong base, such as an alkoxide, to form substituted isoquinolines. First described in 1900 by chemists Siegmund Gabriel and James Colman, this rearrangement, a ring expansion, is seen to be general if there is an enolizable hydrogen on the group attached to the nitrogen, since it is necessary for the nitrogen to abstract a hydrogen to form the carbanion that will close the ring. As shown in the case of the general example below, X is either CO or SO2.

The Nierenstein reaction is an organic reaction describing the conversion of an acid chloride into a haloketone with diazomethane. It is an insertion reaction in that the methylene group from the diazomethane is inserted into the carbon-chlorine bond of the acid chloride.
The Steglich esterification is a variation of an esterification with dicyclohexylcarbodiimide as a coupling reagent and 4-dimethylaminopyridine as a catalyst. The reaction was first described by Wolfgang Steglich in 1978. It is an adaptation of an older method for the formation of amides by means of DCC (dicyclohexylcarbodiimide) and 1-hydroxybenzotriazole (HOBT).
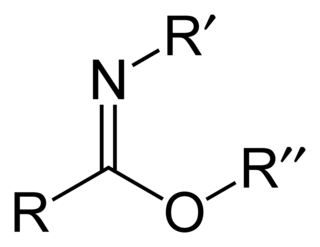
Carboximidates are organic compounds, which can be thought of as esters formed between a imidic acid and an alcohol, with the general formula R-C(=NR')OR".

α,β-Unsaturated carbonyl compounds are organic compounds with the general structure (O=CR)−Cα=Cβ-R. Such compounds include enones and enals, but also carboxylic acids and the corresponding esters and amides. In these compounds, the carbonyl group is conjugated with an alkene. Unlike the case for carbonyls without a flanking alkene group, α,β-unsaturated carbonyl compounds are susceptible to attack by nucleophiles at the β-carbon. This pattern of reactivity is called vinylogous. Examples of unsaturated carbonyls are acrolein (propenal), mesityl oxide, acrylic acid, and maleic acid. Unsaturated carbonyls can be prepared in the laboratory in an aldol reaction and in the Perkin reaction.