
In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula CnH2n-2. Alkynes are traditionally known as acetylenes, although the name acetylene also refers specifically to C2H2, known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic.
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons.

Organolithium reagents are organometallic compounds that contain carbon–lithium bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric.
A non-Kekulé molecule is a conjugated hydrocarbon that cannot be assigned a classical Kekulé structure.
Arynes and benzynes are highly reactive species derived from an aromatic ring by removal of two substituents. Arynes are examples of didehydroarenes, although 1,3- and 1,4-didehydroarenes are also known. Arynes are examples of strained alkynes.
An alkyne trimerisation reaction is a [2+2+2] cycloaddition reaction in which three alkyne units react to form a benzene ring. The reaction requires a metal catalyst. The process is of historic interest as well as being applicable to organic synthesis. Being a cycloaddition reaction, it has high atom economy. Many variations have been developed including cyclisation of mixtures of alkynes and alkenes as well as alkynes and nitriles.

The Michaelis–Arbuzov reaction is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the Arbuzov reaction; phosphite esters (1) react to form phosphonates (2), phosphonites (3) react to form phosphinates (4) and phosphinites (5) react to form phosphine oxides (6).
Aromatization is a chemical reaction in which an aromatic system is formed from a single nonaromatic precursor. Typically aromatization is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene. Aromatization includes the formation of heterocyclic systems.
The Shapiro reaction or tosylhydrazone decomposition is an organic reaction in which a ketone or aldehyde is converted to an alkene through an intermediate hydrazone in the presence of 2 equivalents of organolithium reagent. The reaction was discovered by Robert H. Shapiro in 1967. The Shapiro reaction was used in the Nicolaou Taxol total synthesis. This reaction is very similar to the Bamford–Stevens reaction, which also involves the basic decomposition of tosyl hydrazones.
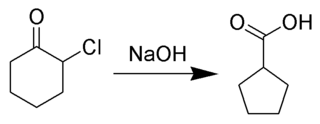
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This rearrangement takes place in the presence of a base, sometimes hydroxide, to yield a carboxylic acid but most of the time either an alkoxide base or an amine to yield an ester or an amide, respectively. α,α'-Dihaloketones eliminate HX under the reaction conditions to give α,β-unsaturated carbonyl compounds.

The calicheamicins are a class of enediyne antitumor antibiotics derived from the bacterium Micromonospora echinospora, with calicheamicin γ1 being the most notable. It was isolated originally in the mid-1980s from the chalky soil, or "caliche pits", located in Kerrville, Texas. The sample was collected by a scientist working for Lederle Labs. It is extremely toxic to all cells and, in 2000, a CD33 antigen-targeted immunoconjugate N-acetyl dimethyl hydrazide calicheamicin was developed and marketed as targeted therapy against the non-solid tumor cancer acute myeloid leukemia (AML). A second calicheamicin-linked monoclonal antibody, inotuzumab ozogamicin an anti-CD22-directed antibody-drug conjugate, was approved by the U.S. Food and Drug Administration on August 17, 2017, for use in the treatment of adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Calicheamicin γ1 and the related enediyne esperamicin are the two of the most potent antitumor agents known.
Robert George Bergman is an American chemist. He is Professor of the Graduate School and Gerald E. K. Branch Distinguished Professor Emeritus at the University of California, Berkeley.

Enediynes are organic compounds containing two triple bonds and one double bond.

The Birch reduction is an organic reaction that is used to convert arenes to cyclohexadienes. The reaction is named after the Australian chemist Arthur Birch and involves the organic reduction of aromatic rings in liquid ammonia with sodium, lithium, or potassium and an alcohol, such as ethanol and tert-butanol. This reaction is unlike catalytic hydrogenation, which usually reduces the aromatic ring all the way to a cyclohexane.
In organic chemistry, enone–alkene cycloadditions are a version of the [2+2] cycloaddition This reaction involves an enone and alkene as substrates. Although the concerted photochemical [2+2] cycloaddition is allowed, the reaction between enones and alkenes is stepwise and involves discrete diradical intermediates.

Hydrogen-bond catalysis is a type of organocatalysis that relies on use of hydrogen bonding interactions to accelerate and control organic reactions. In biological systems, hydrogen bonding plays a key role in many enzymatic reactions, both in orienting the substrate molecules and lowering barriers to reaction. However, chemists have only recently attempted to harness the power of using hydrogen bonds to perform catalysis, and the field is relatively undeveloped compared to research in Lewis acid catalysis.

Transition metal benzyne complexes are organometallic complexes that contain benzyne ligands (C6H4). Unlike benzyne itself, these complexes are less reactive although they undergo a number of insertion reactions.
Free radical damage to DNA can occur as a result of exposure to ionizing radiation or to radiomimetic compounds. Damage to DNA as a result of free radical attack is called indirect DNA damage because the radicals formed can diffuse throughout the body and affect other organs. Malignant melanoma can be caused by indirect DNA damage because it is found in parts of the body not exposed to sunlight. DNA is vulnerable to radical attack because of the very labile hydrogens that can be abstracted and the prevalence of double bonds in the DNA bases that radicals can easily add to.

Kedarcidin is a chromoprotein antitumor antibiotic first isolated from an Actinomycete in 1992, comprising an ansa-bridged enediyne chromophore (shown) as well as an apoprotein that serves to stabilize the toxin in the Actinomycete. Like other members of the enediyne class of drugs—so named for the nine-or-ten-membered core structure bearing an alkene directly attached to two alkynyl appendages—kedarcidin was likely evolved to kill bacteria that compete with the producing organism. Because it achieves this by causing DNA damage, however, kedarcidin is capable of harming tumor cells, as well. Kedarcidin is thus the subject of scientific research, both for its structural complexity as well as its anticancer properties.
In organic chemistry, the hexadehydro-Diels–Alder (HDDA) reaction is an organic chemical reaction between a diyne and an alkyne to form a reactive benzyne species, via a [4+2] cycloaddition reaction. This benzyne intermediate then reacts with a suitable trapping agent to form a substituted aromatic product. This reaction is a derivative of the established Diels–Alder reaction and proceeds via a similar [4+2] cycloaddition mechanism. The HDDA reaction is particularly effective for forming heavily functionalized aromatic systems and multiple ring systems in one synthetic step.













