In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (−OH) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, C
6H
5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.
The von Richter reaction, also named von Richter rearrangement, is a name reaction in the organic chemistry. It is named after Victor von Richter, who discovered this reaction in year 1871. It is the reaction of aromatic nitro compounds with potassium cyanide in aqueous ethanol to give the product of cine substitution by a carboxyl group. Although it is not generally synthetically useful due to the low chemical yield and formation of numerous side products, its mechanism was of considerable interest, eluding chemists for almost 100 years before the currently accepted one was proposed.
In organic chemistry, the Arndt–Eistert reaction is the conversion of a carboxylic acid to its homologue. Named for the German chemists Fritz Arndt (1885–1969) and Bernd Eistert (1902–1978), the method entails treating an acid chlorides with diazomethane. It is a popular method of producing β-amino acids from α-amino acids.

The Scholl reaction is a coupling reaction between two arene compounds with the aid of a Lewis acid and a protic acid. It is named after its discoverer, Roland Scholl, a Swiss chemist.
The Emde degradation is a method for the reduction of a quaternary ammonium cation to a tertiary amine with sodium amalgam:
The Conrad–Limpach synthesis is the condensation of anilines (1) with β-ketoesters (2) to form 4-hydroxyquinolines (4) via a Schiff base (3). The overall reaction type is a combination of both an addition reaction as well as a rearrangement reaction. This reaction was discovered by Max Conrad (1848–1920) and Leonhard Limpach (1852–1933) in 1887 while they were studying the synthesis of quinoline derivatives.

The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat, and the catalyst is an acid like hydrochloric acid.
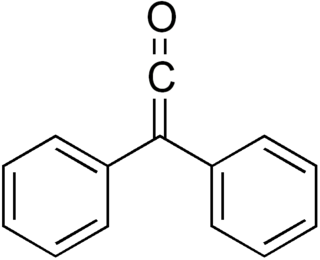
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure. Due to the successive double bonds in the ketene structure R1R2C=C=O, diphenyl ketene is a heterocumule. The most important reaction of diphenyl ketene is the [2+2] cycloaddition at C-C, C-N, C-O, and C-S multiple bonds.

The Westphalen–Lettré rearrangement is a classic organic reaction in organic chemistry describing a rearrangement reaction of cholestane-3β,5α,6β-triol diacetate with acetic anhydride and sulfuric acid. In this reaction one equivalent of water is lost, a double bond is formed at C10–C11 and importantly the methyl group at the C10 position migrates to the C5 position.
The Castro–Stephens coupling is a cross coupling reaction between a copper(I) acetylide and an aryl halide in pyridine, forming a disubstituted alkyne and a copper(I) halide.
The Hofmann–Löffler reaction (also referred to as Hofmann–Löffler–Freytag reaction, Löffler–Freytag reaction, Löffler–Hofmann reaction, as well as Löffler's method) is an organic reaction in which a cyclic amine 2 (pyrrolidine or, in some cases, piperidine) is generated by thermal or photochemical decomposition of N-halogenated amine 1 in the presence of a strong acid (concentrated sulfuric acid or concentrated CF3CO2H). The Hofmann–Löffler–Freytag reaction proceeds via an intramolecular hydrogen atom transfer to a nitrogen-centered radical and is an example of a remote intramolecular free radical C–H functionalization.

Pulvinone, an organic compound belonging to the esters, lactones, alcohols and butenolides classes, is a yellow crystalline solid. Although the pulvinone is not a natural product, several naturally occurring hydroxylated derivatives are known. These hydroxylated pulvinones are produced by fungal species, such as the in Europe common Larch Bolete, or by moulds such as Aspergillus terreus.
Heinrich Hubert Maria Josef Houben was a German chemist. He made achievements within ketone synthesis, terpenes, and camphor studies. After being wounded several times on the front lines in World War I, Houben was made head of the war laboratory. He improved the Hoesch reaction which is now normally called Houben-Hoesch reaction. Houben organized and made a major rework of the book Methods of Organic Chemistry which is now referred to as Houben-Weyl Methods of Organic Chemistry.
The Hinsberg oxindole synthesis is a method of preparing oxindoles from the bisulfite additions of glyoxal. It is named after its inventor Oscar Hinsberg.
The Hinsberg reaction is a chemical test for the detection of primary, secondary and tertiary amines. The reaction was first described by Oscar Hinsberg in 1890. In this test, the amine is shaken well with the Hinsberg reagent in the presence of aqueous alkali. A primary amine will form a soluble sulfonamide salt. Acidification of this salt then precipitates the sulfonamide of the primary amine. A secondary amine in the same reaction will directly form an insoluble sulfonamide. A tertiary amine will not react with the original reagent and will remain insoluble. After adding dilute acid this insoluble amine is converted to a soluble ammonium salt. In this way the reaction can distinguish between the three types of amines.
The Kauffmann olefination is a chemical reaction to convert aldehydes and ketones to olefins with a terminal methylene group. This reaction was discovered by the German chemist Thomas Kauffmann and is related to the better known Tebbe olefination or Wittig reaction.
Fritz Weigert was a German physical chemist. Weigert has made major contributions in the field of photochemistry. He was born in Berlin. He was the nephew of both Karl Weigert and Paul Ehrlich. He was married to Margarete Behmer. Around 1908, he began teaching and conducting research at Berlin University - after studying there. He was a photochemistry professor at Leipzig University from 1914 until being, like other Jewish scientists, forced out by the Nazis in 1934. On January 1, 1935, he immigrated to England and in 1936 was director of the Physiochemical Department of the Cancer Research Institute at Mount Vernon Hospital, Northwood.
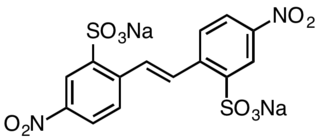
Disodium 4,4′-dinitrostilbene-2,2′-disulfonate is an organic compound with the formula (O2NC6H3(SO3Na)CH)2. This salt is a common precursor to a variety of textile dyes and optical brighteners

Willy Marckwald was a German chemist. </ref>
Tellurium nitride describes chemical compounds of Te containing N3−. Efforts have been made toward the binary nitrides but the results are inconclusive and it appears that such materials are unstable. Still unconfirmed is Te4N4, which would be an analogue of tetraselenium tetranitride (Se4N4) and tetrasulfur tetranitride (S4N4). It has long been known that ammonia reacts with tellurium tetrachloride, which is similar to the method of synthesis of S4N4. The reaction of TeCl4 with a THF solution of N(SiMe3)3 gives a well-defined tellurium nitride [Te6N8(TeCl2)4(THF)4].