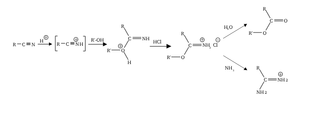
The Pinner reaction refers to the acid catalysed reaction of a nitrile with an alcohol to form an imino ester salt ; this is sometimes referred to as a Pinner salt. The reaction is named after Adolf Pinner, who first described it in 1877. Pinner salts are themselves reactive and undergo additional nucleophilic additions to give various useful products:
Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula C4H4NH. It is a colorless volatile liquid that darkens readily upon exposure to air. Substituted derivatives are also called pyrroles, e.g., N-methylpyrrole, C4H4NCH3. Porphobilinogen, a trisubstituted pyrrole, is the biosynthetic precursor to many natural products such as heme.

An aldol condensation is a condensation reaction in organic chemistry in which an enol or an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by dehydration to give a conjugated enone.
The Fischer indole synthesis is a chemical reaction that produces the aromatic heterocycle indole from a (substituted) phenylhydrazine and an aldehyde or ketone under acidic conditions. The reaction was discovered in 1883 by Emil Fischer. Today antimigraine drugs of the triptan class are often synthesized by this method.
The Michael reaction or Michael addition is the nucleophilic addition of a carbanion or another nucleophile to an α,β-unsaturated carbonyl compound. It belongs to the larger class of conjugate additions. This is one of the most useful methods for the mild formation of C–C bonds. Many asymmetric variants exist.
The Knoevenagel condensation reaction is an organic reaction named after Emil Knoevenagel. It is a modification of the aldol condensation.
The Claisen condensation is a carbon–carbon bond forming reaction that occurs between two esters or one ester and another carbonyl compound in the presence of a strong base, resulting in a β-keto ester or a β-diketone. It is named after Rainer Ludwig Claisen, who first published his work on the reaction in 1887.
The Japp–Klingemann reaction is a chemical reaction used to synthesize hydrazones from β-keto-acids and aryl diazonium salts. The Reaction is named after the chemists Francis Robert Japp and Felix Klingemann.
The Reformatsky reaction is an organic reaction which condenses aldehydes or ketones, with α-halo esters, using a metallic zinc to form β-hydroxy-esters:
The Hantzsch pyridine synthesis or Hantzsch dihydropyridine synthesis is a multi-component organic reaction between an aldehyde such as formaldehyde, 2 equivalents of a β-keto ester such as ethyl acetoacetate and a nitrogen donor such as ammonium acetate or ammonia. The initial reaction product is a dihydropyridine which can be oxidized in a subsequent step to a pyridine. The driving force for this second reaction step is aromatization. This reaction was reported in 1881 by Arthur Rudolf Hantzsch.

The Feist–Benary synthesis is an organic reaction between α-halogen ketones and β-dicarbonyl compounds to produce substituted furan compounds. This condensation reaction is catalyzed by amines such as ammonia and pyridine. The first step in the ring synthesis is related to the Knoevenagel condensation. In the second step the enolate displaces an alkyl halogen in a nucleophilic aliphatic substitution.

The Reed reaction is a chemical reaction that utilizes light to oxidize hydrocarbons to sulfonyl chlorides. The reaction performs via the free radicals. First, the light initiates homolysis of a molecule of chlorine. Then a chlorine atom produced attacks the hydrocarbon chain to form hydrogen chloride and an alkyl free radical. Then SO2 as an electron donor attacks the alkyl radical forming a sulfonyl radical. Finally, the least one attacks another chlorine molecule to produce a sulfonyl chloride and a new chlorine atom which continues the reaction chain.
The Rosenmund reduction is a hydrogenation process in which an acyl chloride is selectively reduced to an aldehyde. The reaction was named after Karl Wilhelm Rosenmund, who first reported it in 1918.
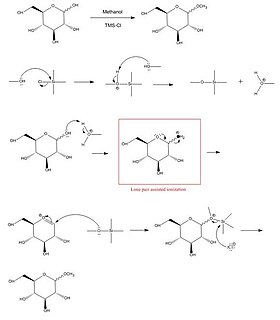
Fischer glycosidation refers to the formation of a glycoside by the reaction of an aldose or ketose with an alcohol in the presence of an acid catalyst. The reaction is named after the German chemist, Emil Fischer, winner of the Nobel Prize in chemistry, 1902, who developed this method between 1893 and 1895.
The Schotten–Baumann reaction is a method to synthesise amides from amines and acid chlorides:
The Hoesch reaction or Houben–Hoesch reaction is an organic reaction in which a nitrile reacts with an arene compound to form an aryl ketone. The reaction is a type of Friedel-Crafts acylation with hydrogen chloride and a Lewis acid catalyst.
Heinrich Hubert Maria Josef Houben was a German chemist. He made achievements within ketone synthesis, terpenes, and camphor studies. After being wounded several times on the front lines in World War I, Houben was made head of the war laboratory. He improved the Hoesch reaction which is now normally called Houben-Hoesch reaction. Houben organized and made a major rework of the book Methods of Organic Chemistry which is now referred to as Houben-Weyl Methods of Organic Chemistry.

Conhydrine is a poisonous alkaloid found in poison hemlock in small quantities.
The Buchner–Curtius–Schlotterbeck reaction is the reaction of aldehydes or ketones with aliphatic diazoalkanes to form homologated ketones. It was first described by Eduard Buchner and Theodor Curtius in 1885 and later by Fritz Schlotterbeck in 1907. Two German chemists also preceded Schlotterbeck in discovery of the reaction, Hans von Pechmann in 1895 and Viktor Meyer in 1905. The reaction has since been extended to the synthesis of β-keto esters from the condensation between aldehydes and diazo esters. The general reaction scheme is as follows:
Werner Zerweck was a German chemist, inventor and industrial leader, who served as CEO of the chemical and pharmaceutical company Cassella from 1953 to 1963. Under his leadership the company focused increasingly on pharmaceuticals and cosmetics rather than its former primary focus, dyes. He was also a member of the advisory board of Deutsche Bank from 1953. Zerweck was one of the pioneers in the development of synthetic fibers.








