 | |
 | |
| Names | |
|---|---|
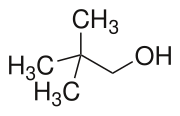
| Preferred IUPAC name 2,2-Dimethylpropan-1-ol | |
| Other names tert-Butyl carbinol tert-Butylmethanol Neoamyl alcohol Neopentanol | |
| Identifiers | |
3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.826 |
| EC Number |
|
PubChem CID | |
| UNII | |
| UN number | 1325 |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C5H12O | |
| Molar mass | 88.150 g·mol−1 |
| Density | 0.812 g/mL at 20 °C |
| Melting point | 52.5 °C (126.5 °F; 325.6 K) |
| Boiling point | 113.5 °C (236.3 °F; 386.6 K) |
| 36 g/L | |
| Solubility | very soluble in ethanol, diethyl ether |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) | −399.4 kJ·mol−1 |
| Hazards | |
| GHS labelling: [2] | |
  | |
| Warning | |
| H226, H228, H319, H332, H335 | |
| P210, P233, P240, P241, P242, P243, P261, P264+P265, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P317, P319, P337+P317, P370+P378, P403+P233, P403+P235, P405, P501 | |
| Flash point | 37 °C (99 °F; 310 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Neopentyl alcohol is a compound with formula (CH3)3CCH2OH. It is a colorless solid. The compound is one of the eight isomers of pentyl alcohol.