
Blue cheese is any of a wide range of cheeses made with the addition of cultures of edible molds, which create blue-green spots or veins through the cheese. Blue cheeses vary in taste from very mild to strong, and from slightly sweet to salty or sharp; in colour from pale to dark; and in consistency from liquid or very soft to firm or hard. They may have a distinctive smell, either from the mold or from various specially cultivated bacteria such as Brevibacterium linens.

Castoreum is a yellowish exudate from the castor sacs of mature beavers. Beavers use castoreum in combination with urine to scent mark their territory. Both beaver sexes have a pair of castor sacs and a pair of anal glands, located in two cavities under the skin between the pelvis and the base of the tail. The castor sacs are not true glands on a cellular level, hence references to these structures as preputial glands, castor glands, or scent glands are misnomers.

The Controlled Drugs and Substances Act is Canada's federal drug control statute. Passed in 1996 under Prime Minister Jean Chrétien's government, it repeals the Narcotic Control Act and Parts III and IV of the Food and Drugs Act, and establishes eight Schedules of controlled substances and two Classes of precursors. It provides that "The Governor in Council may, by order, amend any of Schedules I to VIII by adding to them or deleting from them any item or portion of an item, where the Governor in Council deems the amendment to be necessary in the public interest."
The Bouveault–Blanc reduction is a chemical reaction in which an ester is reduced to primary alcohols using absolute ethanol and sodium metal. It was first reported by Louis Bouveault and Gustave Louis Blanc in 1903. Bouveault and Blanc demonstrated the reduction of ethyl oleate and n-butyl oleate to oleyl alcohol. Modified versions of which were subsequently refined and published in Organic Syntheses.

The garden strawberry is a widely grown hybrid species of the genus Fragaria, collectively known as the strawberries, which are cultivated worldwide for their fruit. The fruit is widely appreciated for its characteristic aroma, bright red color, juicy texture, and sweetness. It is consumed in large quantities, either fresh or in such prepared foods as jam, juice, pies, ice cream, milkshakes, and chocolates. Artificial strawberry flavorings and aromas are also widely used in products such as candy, soap, lip gloss, perfume, and many others.

1-Hexene (hex-1-ene) is an organic compound with the formula C6H12. It is an alkene that is classified in industry as higher olefin and an alpha-olefin, the latter term meaning that the double bond is located at the alpha (primary) position, endowing the compound with higher reactivity and thus useful chemical properties. 1-Hexene is an industrially significant linear alpha olefin. 1-Hexene is a colourless liquid.
Heptanol may refer to any of four isomeric chemical compounds:

Harpegnathos saltator, sometimes called the Indian jumping ant or Jerdon's jumping ant, is a species of ant found in India. They have long mandibles and have the ability to leap a few inches. They are large-eyed and active predators that hunt mainly in the early morning. The colonies are small and the difference between workers and queens is very slight.
Heptyl acetate (C9H18O2), also known as acetate C-7, is a colorless alcohol-soluble liquid that is the ester formed by the condensation of 1-heptanol and acetic acid.

The bioconversion of biomass to mixed alcohol fuels can be accomplished using the MixAlco process. Through bioconversion of biomass to a mixed alcohol fuel, more energy from the biomass will end up as liquid fuels than in converting biomass to ethanol by yeast fermentation.
The Guerbet reaction, named after Marcel Guerbet (1861–1938), is an organic reaction that converts a primary alcohol into its β-alkylated dimer alcohol with loss of one equivalent of water. The process is of interest because it converts simple inexpensive feedstocks into more valuable products. Its main disadvantage is that the reaction produces mixtures.

In chemistry, a sulfenic acid is an organosulfur compound and oxoacid with the general formula R−S−OH. It is the first member of the family of organosulfur oxoacids, which also include sulfinic acids and sulfonic acids, respectively. The base member of the sulfenic acid series with R = H is hydrogen thioperoxide.

Olfactory receptor 2W1 is a protein that in humans is encoded by the OR2W1 gene.

Olfactory receptor 2J2 is a protein that in humans is encoded by the OR2J2 gene.

The snow leopard, commonly known as the ounce, is a species of large cat in the genus Panthera of the family Felidae. The species is native to the mountain ranges of Central and South Asia. It is listed as Vulnerable on the IUCN Red List because the global population is estimated to number fewer than 10,000 mature individuals and is expected to decline about 10% by 2040. It is mainly threatened by poaching and habitat destruction following infrastructural developments. It inhabits alpine and subalpine zones at elevations of 3,000–4,500 m (9,800–14,800 ft), ranging from eastern Afghanistan, the Himalayas and the Tibetan Plateau to southern Siberia, Mongolia and western China. In the northern part of its range, it also lives at lower elevations.

2-Heptanol is a chemical compound which is an isomer of heptanol. It is a secondary alcohol with the hydroxyl on the second carbon of the straight seven-carbon chain.
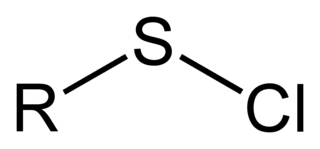
In organosulfur chemistry, a sulfenyl chloride is a functional group with the connectivity R−S−Cl, where R is alkyl or aryl. Sulfenyl chlorides are reactive compounds that behave as sources of RS+. They are used in the formation of RS−N and RS−O bonds. According to IUPAC nomenclature they are named as alkyl thiohypochlorites, i.e. esters of thiohypochlorous acid.

Contraction band necrosis is a type of uncontrolled cell death (necrosis) unique to cardiac myocytes and thought to arise in reperfusion from hypercontraction, which results in sarcolemmal rupture.

4-Heptanone or heptan-4-one is an organic compound with the formula (CH3CH2CH2)2CO. It is a colorless liquid.

Apocephalus paraponerae is a species of fly in the family Phoridae discovered by Borgmeier in 1958. This species is a parasitoid of the giant tropical ant Paraponera clavata and uses both visual and chemical cues to locate its host. A. paraponerae can locate fighting or injured ants through host-produced alarm pheromones. Female flies are attracted to the ant to feed and oviposit, while males are attracted to feed and locate females for mating. There is some evidence that suggests that A. paraponerae is a cryptic species complex of at least four genetically distinct species.
















