
In organic chemistry, an alkane, or paraffin, is an acyclic saturated hydrocarbon. In other words, an alkane consists of hydrogen and carbon atoms arranged in a tree structure in which all the carbon–carbon bonds are single. Alkanes have the general chemical formula CnH2n+2. The alkanes range in complexity from the simplest case of methane, where n = 1, to arbitrarily large and complex molecules, like pentacontane or 6-ethyl-2-methyl-5-(1-methylethyl) octane, an isomer of tetradecane.

In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

Ethanol is an organic compound with the chemical formula CH3CH2OH. It is an alcohol, with its formula also written as C2H5OH, C2H6O or EtOH, where Et stands for ethyl. Ethanol is a volatile, flammable, colorless liquid with a characteristic wine-like odor and pungent taste. As a psychoactive depressant, it is the active ingredient in alcoholic beverages, and the second most consumed drug globally behind caffeine.

In organic chemistry, a thiol, or thiol derivative, is any organosulfur compound of the form R−SH, where R represents an alkyl or other organic substituent. The −SH functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols, and the word is a blend of "thio-" with "alcohol".

Ethylene oxide is an organic compound with the formula C2H4O. It is a cyclic ether and the simplest epoxide: a three-membered ring consisting of one oxygen atom and two carbon atoms. Ethylene oxide is a colorless and flammable gas with a faintly sweet odor. Because it is a strained ring, ethylene oxide easily participates in a number of addition reactions that result in ring-opening. Ethylene oxide is isomeric with acetaldehyde and with vinyl alcohol. Ethylene oxide is industrially produced by oxidation of ethylene in the presence of a silver catalyst.

1,3-Butadiene is the organic compound with the formula CH2=CH-CH=CH2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene.
In organic chemistry, a carbanion is an anion in which carbon is negatively charged.

Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.
Fatty alcohols (or long-chain alcohols) are usually high-molecular-weight, straight-chain primary alcohols, but can also range from as few as 4–6 carbons to as many as 22–26, derived from natural fats and oils. The precise chain length varies with the source. Some commercially important fatty alcohols are lauryl, stearyl, and oleyl alcohols. They are colourless oily liquids (for smaller carbon numbers) or waxy solids, although impure samples may appear yellow. Fatty alcohols usually have an even number of carbon atoms and a single alcohol group (–OH) attached to the terminal carbon. Some are unsaturated and some are branched. They are widely used in industry. As with fatty acids, they are often referred to generically by the number of carbon atoms in the molecule, such as "a C12 alcohol", that is an alcohol having 12 carbons, for example dodecanol.
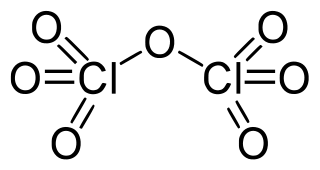
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:

In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles are substantially smaller than the idealized value of approximately 109°. Because of their high strain, the heat of combustion for these small rings is elevated.

In organic chemistry, terminal alkenes are a family of organic compounds which are alkenes with a chemical formula CxH2x, distinguished by having a double bond at the primary, alpha (α), or 1- position. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications.

In organic chemistry, hyperconjugation refers to the delocalization of electrons with the participation of bonds of primarily σ-character. Usually, hyperconjugation involves the interaction of the electrons in a sigma (σ) orbital with an adjacent unpopulated non-bonding p or antibonding σ* or π* orbitals to give a pair of extended molecular orbitals. However, sometimes, low-lying antibonding σ* orbitals may also interact with filled orbitals of lone pair character (n) in what is termed negative hyperconjugation. Increased electron delocalization associated with hyperconjugation increases the stability of the system. In particular, the new orbital with bonding character is stabilized, resulting in an overall stabilization of the molecule. Only electrons in bonds that are in the β position can have this sort of direct stabilizing effect — donating from a sigma bond on an atom to an orbital in another atom directly attached to it. However, extended versions of hyperconjugation can be important as well. The Baker–Nathan effect, sometimes used synonymously for hyperconjugation, is a specific application of it to certain chemical reactions or types of structures.
Perchloryl fluoride is a reactive gas with the chemical formula ClO
3F. It has a characteristic sweet odor that resembles gasoline and kerosene. It is toxic and is a powerful oxidizing and fluorinating agent. It is the acid fluoride of perchloric acid.

Anacardic acids are phenolic lipids, chemical compounds found in the shell of the cashew nut. An acid form of urushiol, they also cause an allergic skin rash on contact, known as urushiol-induced contact dermatitis. Anacardic acid is a yellow liquid. It is partially miscible with ethanol and ether, but nearly immiscible with water. Chemically, anacardic acid is a mixture of several closely related organic compounds. Each consists of a salicylic acid substituted with an alkyl chain that has 15 or 17 carbon atoms. The alkyl group may be saturated or unsaturated; anacardic acid is a mixture of saturated and unsaturated molecules. The exact mixture depends on the species of the plant. The 15-carbon unsaturated side chain compound found in the cashew plant is lethal to Gram-positive bacteria.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula [N(CH2CH3)4]+Cl−, sometimes written as [NEt4]Cl. In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis.
1-Dodecene is an alkene with the formula C10H21CH=CH2, consisting of a chain of twelve carbon atoms ending with a double bond. While there are many isomers of dodecene depending on which carbon the double bond is placed, this isomer is of greater commercial importance. It is classified as an alpha-olefin. Alpha-olefins are distinguished by having a double bond at the primary or alpha (α) position. This location of a double bond enhances the reactivity of the compound and makes it useful for a number of applications, especially for the production of detergents.
In organic chemistry, the Ziegler process is a method for producing fatty alcohols from ethylene using an organoaluminium compound. The reaction produces linear primary alcohols with an even numbered carbon chain. The process uses an aluminum compound to oligomerize ethylene and allow the resulting alkyl group to be oxygenated. The usually targeted products are fatty alcohols, which are otherwise derived from natural fats and oils. Fatty alcohols are used in food and chemical processing. They are useful due to their amphipathic nature. The synthesis route is named after Karl Ziegler, who described the process in 1955.
Radical fluorination is a type of fluorination reaction, complementary to nucleophilic and electrophilic approaches. It involves the reaction of an independently generated carbon-centered radical with an atomic fluorine source and yields an organofluorine compound.
















