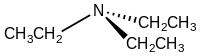
In chemistry, amines are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Formally, amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline. Inorganic derivatives of ammonia are also called amines, such as monochloramine.

Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) molecular ion with the chemical formula NH+4 or [NH4]+. It is formed by the addition of a proton to ammonia. Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations, where one or more hydrogen atoms are replaced by organic or other groups. Not only is ammonium a source of nitrogen and a key metabolite for many living organisms, but it is an integral part of the global nitrogen cycle. As such, human impact in recent years could have an effect on the biological communities that depend on it.

In chemistry, a hydrochloride is an acid salt resulting, or regarded as resulting, from the reaction of hydrochloric acid with an organic base. An alternative name is chlorhydrate, which comes from French. An archaic alternative name is muriate, derived from hydrochloric acid's ancient name: muriatic acid.

Methylamine is an organic compound with a formula of CH3NH2. This colorless gas is a derivative of ammonia, but with one hydrogen atom being replaced by a methyl group. It is the simplest primary amine.

In organic chemistry, quaternary ammonium cations, also known as quats, are positively-charged polyatomic ions of the structure [NR4]+, where R is an alkyl group, an aryl group or organyl group. Unlike the ammonium ion and the primary, secondary, or tertiary ammonium cations, the quaternary ammonium cations are permanently charged, independent of the pH of their solution. Quaternary ammonium salts or quaternary ammonium compounds are salts of quaternary ammonium cations. Polyquats are a variety of engineered polymer forms which provide multiple quat molecules within a larger molecule.

In chemistry, the term phosphonium describes polyatomic cations with the chemical formula PR+
4. These cations have tetrahedral structures. The salts are generally colorless or take the color of the anions.
Morpholine is an organic chemical compound having the chemical formula O(CH2CH2)2NH. This heterocycle features both amine and ether functional groups. Because of the amine, morpholine is a base; its conjugate acid is called morpholinium. For example, treating morpholine with hydrochloric acid generates the salt morpholinium chloride. It is a colorless liquid with a weak, ammonia- or fish-like odor. The naming of morpholine is attributed to Ludwig Knorr, who incorrectly believed it to be part of the structure of morphine.

Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005.

N,N-Diisopropylethylamine, or Hünig's base, is an organic compound that is a tertiary amine. It is named after the German chemist Siegfried Hünig. It is used in organic chemistry as a non-nucleophilic base. It is commonly abbreviated as DIPEA,DIEA, or i-Pr2NEt.
In organic chemistry, the Menshutkin reaction converts a tertiary amine into a quaternary ammonium salt by reaction with an alkyl halide. Similar reactions occur when tertiary phosphines are treated with alkyl halides.

Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately 500,000 tonnes produced in 1998. Ethylenediamine is the first member of the so-called polyethylene amines.
Benzyl chloride, or α-chlorotoluene, is an organic compound with the formula C6H5CH2Cl. This colorless liquid is a reactive organochlorine compound that is a widely used chemical building block.
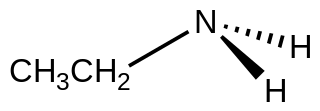
Ethylamine, also known as ethanamine, is an organic compound with the formula CH3CH2NH2. This colourless gas has a strong ammonia-like odor. It condenses just below room temperature to a liquid miscible with virtually all solvents. It is a nucleophilic base, as is typical for amines. Ethylamine is widely used in chemical industry and organic synthesis.

Diethylamine is an organic compound with the formula (CH3CH2)2NH. It is a secondary amine. It is a flammable, weakly alkaline liquid that is miscible with most solvents. It is a colorless liquid, but commercial samples often appear brown due to impurities. It has a strong ammonia-like odor.
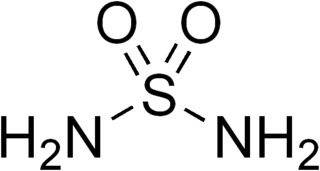
Sulfamide is a compound with the chemical formula SO2(NH2)2 and structure H2N−S(=O)2−NH2. Sulfamide is produced by the reaction of sulfuryl chloride with ammonia. Sulfamide was first prepared in 1838 by the French chemist Henri Victor Regnault.
In chemistry, work-up refers to the series of manipulations required to isolate and purify the product(s) of a chemical reaction. The term is used colloquially to refer to these manipulations, which may include:

Potassium tetrachloroplatinate(II) is the chemical compound with the formula K2PtCl4. This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42−. Related salts are also known including Na2PtCl4, which is brown-colored and soluble in alcohols, and quaternary ammonium salts, which are soluble in a broader range of organic solvents.

Tetramethylammonium chloride is one of the simplest quaternary ammonium salts, with four methyl groups tetrahedrally attached to the central N. The chemical formula (CH3)4N+Cl− is often abbreviated further as Me4N+Cl−. It is a hygroscopic colourless solid that is soluble in water and polar organic solvents. Tetramethylammonium chloride is a major industrial chemical, being used widely as a chemical reagent and also as a low-residue bactericide in such processes as hydrofracking. In the laboratory, it has fewer synthetic chemical applications than quaternary ammonium salts containing longer N-alkyl substituents, which are used extensively as phase-transfer catalysts.

Tetraethylammonium chloride (TEAC) is a quaternary ammonium compound with the chemical formula [N(CH2CH3)4]+Cl−, sometimes written as [NEt4]Cl. In appearance, it is a hygroscopic, colorless, crystalline solid. It has been used as the source of tetraethylammonium ions in pharmacological and physiological studies, but is also used in organic chemical synthesis.
In chemistry, a fatty amine is loosely defined as any amine possessing a mostly linear hydrocarbon chain of eight or more carbon atoms. They are typically prepared from the more abundant fatty acids, with vegetable or seed-oils being the ultimate starting material. As such they are often mixtures of chain lengths, ranging up to about C22. They can be classified as oleochemicals. Commercially important members include coco amine, oleylamine, tallow amine, and soya amine. These compounds and their derivatives are used as fabric softeners, froth flotation agents, corrosion inhibitors, lubricants and friction modifiers. They are also the basis for a variety of cosmetic formulations.