
Lewisite (L) (A-243) is an organoarsenic compound. It was once manufactured in the U.S., Japan, Germany and the Soviet Union for use as a chemical weapon, acting as a vesicant and lung irritant. Although the substance is colorless and odorless in its pure form, impure samples of lewisite are a yellow, brown, violet-black, green, or amber oily liquid with a distinctive odor that has been described as similar to geraniums.
Salt water aspiration syndrome is a rare diving disorder suffered by scuba divers who inhale a mist of seawater, usually from a faulty demand valve, causing irritation of the lungs. It is not the same thing as aspiration of salt water as a bulk liquid, i.e. drowning. It can usually be treated by rest for several hours. If severe, medical assessment is required. First described by Carl Edmonds.

A blister agent, is a chemical compound that causes severe skin, eye and mucosal pain and irritation. They are named for their ability to cause severe chemical burns, resulting in painful water blisters on the bodies of those affected. Although the term is often used in connection with large-scale burns caused by chemical spills or chemical warfare agents, some naturally occurring substances such as cantharidin are also blister-producing agents (vesicants). Furanocoumarin, another naturally occurring substance, causes vesicant-like effects indirectly, for example, by increasing skin photosensitivity greatly. Vesicants have medical uses including wart removal but can be dangerous if even small amounts are ingested.

A chemical burn occurs when living tissue is exposed to a corrosive substance or a cytotoxic agent. Chemical burns follow standard burn classification and may cause extensive tissue damage. The main types of irritant and/or corrosive products are: acids, bases, oxidizers / reducing agents, solvents, and alkylants. Additionally, chemical burns can be caused by biological toxins and by some types of cytotoxic chemical weapons, e.g., vesicants such as mustard gas and Lewisite, or urticants such as phosgene oxime.

Chlormethine, also known as mechlorethamine, mustine, HN2, and embikhin (эмбихин), is a nitrogen mustard sold under the brand name Mustargen among others. It is the prototype of alkylating agents, a group of anticancer chemotherapeutic drugs. It works by binding to DNA, crosslinking two strands and preventing cell duplication. It binds to the N7 nitrogen on the DNA base guanine. As the chemical is a blister agent, its use is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Phosgene oxime, or CX, is an organic compound with the formula Cl2CNOH. It is a potent chemical weapon, specifically a nettle agent, which is a type of blister agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable and irritating odor. It is used as a reagent in organic chemistry.

Abrin is an extremely toxic toxalbumin found in the seeds of the rosary pea, Abrus precatorius. It has a median lethal dose of 0.7 micrograms per kilogram of body mass when given to mice intravenously. The median toxic dose for humans ranges from 10 to 1000 micrograms per kilogram when ingested and is 3.3 micrograms per kilogram when inhaled.

Pneumonitis describes general inflammation of lung tissue. Possible causative agents include radiation therapy of the chest, exposure to medications used during chemo-therapy, the inhalation of debris, aspiration, herbicides or fluorocarbons and some systemic diseases. If unresolved, continued inflammation can result in irreparable damage such as pulmonary fibrosis.
Acute severe asthma, also known as status asthmaticus, is an acute exacerbation of asthma that does not respond to standard treatments of bronchodilators (inhalers) and corticosteroids. Asthma is caused by multiple genes, some having protective effect, with each gene having its own tendency to be influenced by the environment although a genetic link leading to acute severe asthma is still unknown. Symptoms include chest tightness, rapidly progressive dyspnea, dry cough, use of accessory respiratory muscles, fast and/or labored breathing, and extreme wheezing. It is a life-threatening episode of airway obstruction and is considered a medical emergency. Complications include cardiac and/or respiratory arrest. The increasing prevalence of atopy and asthma remains unexplained but may be due to infection with respiratory viruses.

Farmer's lung is a hypersensitivity pneumonitis induced by the inhalation of biologic dusts coming from hay dust or mold spores or any other agricultural products. It results in a type III hypersensitivity inflammatory response and can progress to become a chronic condition which is considered potentially dangerous.
Occupational lung diseases comprise a broad group of diseases, including occupational asthma, industrial bronchitis, chronic obstructive pulmonary disease (COPD), bronchiolitis obliterans, inhalation injury, interstitial lung diseases, infections, lung cancer and mesothelioma. These can be caused directly or due to immunological response to an exposure to a variety of dusts, chemicals, proteins or organisms. Occupational cases of interstitial lung disease may be misdiagnosed as COPD, idiopathic pulmonary fibrosis, or a myriad of other diseases; leading to a delay in identification of the causative agent.

2-Chloro-N,N-bis(2-chloroethyl)ethanamine, also known as trichlormethine, tris(2-chloroethyl)amine is the organic compound with the formula N(CH2CH2Cl)3. Often abbreviated HN3 or HN-3, it is a powerful blister agent and a nitrogen mustard used for chemical warfare. HN3 was the last of the nitrogen mustard agents developed. It was designed as a military agent and is the only one of the nitrogen mustards that is still used for military purposes. It is the principal representative of the nitrogen mustards because its vesicant properties are almost equal to those of HD and thus the analogy between the two types of mustard is the strongest. As a vesicant the use and production is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
Potassium nitrate is an oxidizer so storing it near fire hazards or reducing agents should be avoided to minimise risk in case of a fire.

Bird fancier's lung (BFL), also known as bird breeder's lung, is a type of hypersensitivity pneumonitis. It can cause shortness of breath, fever, dry cough, chest pain, anorexia and weight loss, fatigue, and progressive pulmonary fibrosis. It is triggered by exposure to avian proteins present in the dry dust of droppings or feathers of a variety of birds. The lungs become inflamed, with granuloma formation. It mostly affects people who work with birds or own many birds.

Phenyldichloroarsine, also known by its wartime name phenyl Dick and its NATO abbreviation PD, is an organic arsenical vesicant and vomiting agent developed by Germany and France for use as a chemical warfare agent during World War I. The agent is known by multiple synonyms and is technically classified as a vesicant, or blister agent.
Acute inhalation injury may result from frequent and widespread use of household cleaning agents and industrial gases. The airways and lungs receive continuous first-pass exposure to non-toxic and irritant or toxic gases via inhalation. Irritant gases are those that, on inhalation, dissolve in the water of the respiratory tract mucosa and provoke an inflammatory response, usually from the release of acidic or alkaline radicals. Smoke, chlorine, phosgene, sulfur dioxide, hydrogen chloride, hydrogen sulfide, nitrogen dioxide, ozone, and ammonia are common irritants.
Chlorine gas poisoning is an illness resulting from the effects of exposure to chlorine beyond the threshold limit value.

Nitrogen dioxide poisoning is the illness resulting from the toxic effect of nitrogen dioxide. It usually occurs after the inhalation of the gas beyond the threshold limit value. Nitrogen dioxide is reddish-brown with a very harsh smell at high concentrations, at lower concentrations it is colorless but may still have a harsh odour. Nitrogen dioxide poisoning depends on the duration, frequency, and intensity of exposure.
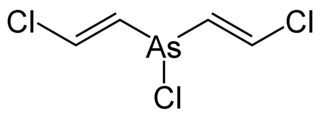
Lewisite 2(L-2) is an organoarsenic chemical weapon with the formula AsCl(CH=CHCl)2. It is similar to lewisite 1 and lewisite 3 and was first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride (lewisite 1) as well as bis(2-chloroethenyl) arsinous chloride (lewisite 2) and tris(2-chlorovinyl)arsine (lewisite 3). Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.

Lewisite 3(L-3) is an organoarsenic chemical weapon like lewisite 1 and lewisite 2 first synthesized in 1904 by Julius Arthur Nieuwland. It is usually found as a mixture of 2-chlorovinylarsonous dichloride as well as bis(2-chloroethenyl) arsinous chloride and tris(2-chlorovinyl)arsine. Pure lewisite 1 is an oily, colorless liquid, however, the impure mixture can appear amber to black with an odor distinct to geraniums.














