 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name Diphenylarsinous chloride | |
| Other names diphenylchlorarsine, diphenyl arsine chloride, diphenylchloroarsenic, chlorodiphenylarsane, sneezing gas | |
| Identifiers | |
3D model (JSmol) | |
| Abbreviations | Ph2AsCl |
| ChemSpider | |
| ECHA InfoCard | 100.010.839 |
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
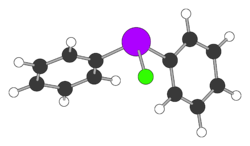
| C12H10AsCl | |
| Molar mass | 264.59 g mol−1 |
| Appearance | colorless crystalline solid |
| Density | 1.55 g/cm3 |
| Melting point | 42 °C (108 °F; 315 K) |
| Boiling point | 307.2 °C (585.0 °F; 580.3 K) |
| −145.5·10−6 cm3/mol | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Diphenylchloroarsine (DA) is the organoarsenic compound with the formula (C6H5)2AsCl. It is highly toxic and was once used in chemical warfare. It is also an intermediate in the preparation of other organoarsenic compounds. The molecule consists of a pyramidal As(III) center attached to two phenyl rings and one chloride. It was also known as sneezing oil during World War I by the Allies.