Related Research Articles

Mouthwash, mouth rinse, oral rinse, or mouth bath is a liquid which is held in the mouth passively or swirled around the mouth by contraction of the perioral muscles and/or movement of the head, and may be gargled, where the head is tilted back and the liquid bubbled at the back of the mouth.

Phenol is an aromatic organic compound with the molecular formula C6H5OH. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group bonded to a hydroxy group. Mildly acidic, it requires careful handling because it can cause chemical burns.
Cresols are a group of aromatic organic compounds. They are widely-occurring phenols which may be either natural or manufactured. They are also categorized as methyl phenols. Cresols commonly occur as either solids or liquids because their melting points are generally close to room temperature. Like other types of phenols, they are slowly oxidized by exposure to air, and the resulting impurities often give the samples a yellow to brownish red tint. Cresols have an odor characteristic to that of other simple phenols, reminiscent to some of a "coal tar" smell. The name "cresol" is an adduct of phenol and their traditional source, creosote.

Rubbing alcohol, also known as surgical spirit in some regions, refers to a group of denatured alcohols commonly used as topical antiseptics. These solutions are primarily composed of either isopropyl alcohol (isopropanol) or ethanol, with isopropyl alcohol being the more widely available formulation. Rubbing alcohol is rendered undrinkable by the addition of bitterants or other denaturants.

A disinfectant is a chemical substance or compound used to inactivate or destroy microorganisms on inert surfaces. Disinfection does not necessarily kill all microorganisms, especially resistant bacterial spores; it is less effective than sterilization, which is an extreme physical or chemical process that kills all types of life. Disinfectants are generally distinguished from other antimicrobial agents such as antibiotics, which destroy microorganisms within the body, and antiseptics, which destroy microorganisms on living tissue. Disinfectants are also different from biocides—the latter are intended to destroy all forms of life, not just microorganisms. Disinfectants work by destroying the cell wall of microbes or interfering with their metabolism. It is also a form of decontamination, and can be defined as the process whereby physical or chemical methods are used to reduce the amount of pathogenic microorganisms on a surface.

Methyl salicylate (oil of wintergreen or wintergreen oil) is an organic compound with the formula C8H8O3. It is the methyl ester of salicylic acid. It is a colorless, viscous liquid with a sweet, fruity odor reminiscent of root beer (in which it is used as a flavoring), but often associatively called "minty", as it is an ingredient in mint candies. It is produced by many species of plants, particularly wintergreens. It is also produced synthetically, used as a fragrance and as a flavoring agent.

Brucine is an alkaloid closely related to strychnine, most commonly found in the Strychnos nux-vomica tree. Brucine poisoning is rare, since it is usually ingested with strychnine, and strychnine is more toxic than brucine. In chemical synthesis, it can be used as a tool for stereospecific chemical syntheses.

The health effects of wine are mainly determined by its active ingredient – alcohol. Preliminary studies found that drinking small quantities of wine, particularly of red wine, may be associated with a decreased risk of cardiovascular diseases, cognitive decline, stroke, diabetes mellitus, metabolic syndrome, and early death. Other studies found no such effects.
Guaiacol is an organic compound with the formula C6H4(OH)(OCH3). It is a phenolic compound containing a methoxy functional group. Guaiacol appears as a viscous colorless oil, although aged or impure samples are often yellowish. It occurs widely in nature and is a common product of the pyrolysis of wood.
Neurolysis is the application of physical or chemical agents to a nerve in order to cause a temporary degeneration of targeted nerve fibers. When the nerve fibers degenerate, it causes an interruption in the transmission of nerve signals. In the medical field, this is most commonly and advantageously used to alleviate pain in cancer patients.

Phorbol is a natural, plant-derived organic compound. It is a member of the tigliane family of diterpenes. Phorbol was first isolated in 1934 as the hydrolysis product of croton oil, which is derived from the seeds of the purging croton, Croton tiglium. The structure of phorbol was determined in 1967. Various esters of phorbol have important biological properties, the most notable of which is the capacity to act as tumor promoters through activation of protein kinase C. They mimic diacylglycerols, glycerol derivatives in which two hydroxyl groups have reacted with fatty acids to form esters. The most common and potent phorbol ester is 12-O-tetradecanoylphorbol-13-acetate (TPA), also called phorbol-12-myristate-13-acetate (PMA), which is used as a biomedical research tool in contexts such as models of carcinogenesis.
A chemical peel is a treatment used to improve and smooth the texture of the skin. The skin on the face is most commonly treated, but peels can also be performed on the body. Chemical peels are intended to remove the outermost layers of the skin. To accomplish this task, the chosen peel solution induces a controlled injury to the skin, which causes the skin to peel. This process leads to smoother skin can improve fine lines, acne scars, and pigment. Medium depth peels must be performed by a medical provider.
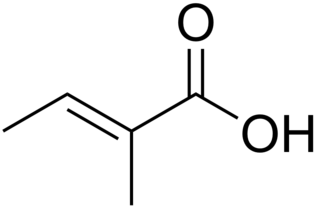
Tiglic acid is a monocarboxylic unsaturated organic acid. It is found in croton oil and in several other natural products. It has also been also isolated from the defensive secretion of certain beetles.

Absinthe is an anise-flavored spirit derived from several plants, including the flowers and leaves of Artemisia absinthium, together with green anise, sweet fennel, and other medicinal and culinary herbs. Historically described as a highly alcoholic spirit, it is 45–74% ABV or 90–148 proof in the US. Absinthe traditionally has a natural green color but may also be colorless. It is commonly referred to in historical literature as la fée verte'the green fairy'. While sometimes casually referred to as a liqueur, absinthe is not traditionally bottled with sugar or sweeteners. Absinthe is traditionally bottled at a high level of alcohol by volume, but it is normally diluted with water before being consumed.

Aplysiatoxin is a cyanotoxin produced by certain cyanobacteria species. It is used as a defensive secretion to protect these cyanobacteria from predation by fish, being a potent irritant and carcinogen, by acting as a powerful activator of protein kinase C. While this action has a tumour-promoting effect, protein kinase C activation can be medically beneficial for some other applications, and synthetic analogues of aplysiatoxin have been researched for anti-cancer effects.

Phorbol 12,13-dibutyrate (PDBu) is a phorbol ester which is one of the constituents of croton oil. As an activator of protein kinase C, it is a weak tumor promoter compared to 12-O-tetradecanoylphorbol-13-acetate.
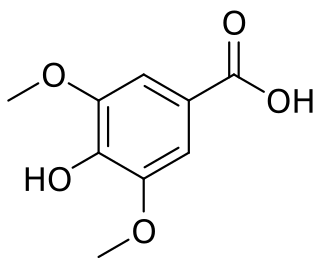
Syringic acid is a naturally occurring phenolic compound and dimethoxybenzene that is commonly found as a plant metabolite.

Catherine "Cathy" Ames, later known as Kate Trask or Kate Albey, is a fictional character and the main antagonist in John Steinbeck's novel East of Eden. She is married to the main protagonist Adam Trask, and the mother of his twin sons, Caleb and Aron. Beneath her charming, attractive facade, she is an evil woman who manipulates and destroys people for her own amusement and profit. Steinbeck characterizes her as a "psychic monster" with a "malformed soul".

Mezerein is a toxic diterpene ester found in the sap of Daphne mezereum and related plants. Plants of the genera Euphorbiaceae and Thymelaeaceae possess a wide variety of different phorbol esters, which share the capacity of mimicking diacylglycerol (DAG) and thus activating different isoforms of protein kinase C. Mezerein was first isolated in 1975. It has antileukemic properties in mice, but it is also defined as a weak promoter of skin cancers in the same species. All parts of the plants contain an acrid and irritant sap that contains mezerein, thought to be the principal poison. The sap is especially prevalent in the bark and berries.
In December 2016, over 70 people died of methanol poisoning in the Russian city of Irkutsk. Precipitated by the consumption of adulterated surrogate alcohol, it was the deadliest such incident in Russia's post-Soviet history.
References
- 1 2 Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica . Vol. 7 (11th ed.). Cambridge University Press. p. 511.
- ↑ Wambier, CG; Lee, KC; Soon, SL; Sterling, JB; Rullan, PP; Landau, M; Brody, HJ; International Peeling, Society. (August 2019). "Advanced chemical peels: Phenol-croton oil peel". Journal of the American Academy of Dermatology. 81 (2): 327–336. doi:10.1016/j.jaad.2018.11.060. PMID 30550827. S2CID 54631945.
- ↑ Orra, S; Waltzman, J. T.; Mlynek, K; Duraes, E. F.; Kundu, N; Zins, J. E. (2015). "Periorbital Phenol-Croton Oil Chemical Peel in Conjunction with Blepharoplasty: An Evolving Technique for Periorbital Facial Rejuvenation". Plastic and Reconstructive Surgery. 136 (4 Suppl): 99–100. doi: 10.1097/01.prs.0000472401.26529.67 . PMID 26397611.
- ↑ Lawrence Kruger, ed. (2001). Methods in Pain Research. CRC Press.
- ↑ Berenblum, I; Shubik, P (1947). "The Role of Croton Oil Applications, Associated with a Single Painting of a Carcinogen, in Tumour Induction of the Mouse's Skin". British Journal of Cancer. 1 (4): 379–382. doi:10.1038/bjc.1947.35. PMC 2007538 . PMID 18906315.
- ↑ Preston, R. D.; Nicolai, E.; Reed, R.; Millard, A. (October 1948). "An Electron Microscope Study of Cellulose in the Wall of Valonia Ventricosa". Nature. 162 (4121): 665–667. Bibcode:1948Natur.162..665P. doi:10.1038/162665a0. PMID 18888170. S2CID 4007842.
- ↑ Meyer-Bertenrath, JG (1969). "150 Years of croton oil research". Experientia. 25 (1): 1–5. doi:10.1007/BF01903855. PMID 4885798. S2CID 26826980.
- ↑ E. Hecker (1967-01-31). "Phorbol esters from croton oil: Chemical nature and biological activities". Naturwissenschaften. 54 (11): 282–4. Bibcode:1967NW.....54..282H. doi:10.1007/BF00620887. PMID 5589922. S2CID 27775957.
- ↑ Ostlund, Mike. Find 'em, chase 'em, sink 'em, Globe Pequot, 2006, p. 88. ISBN 1-59228-862-6
- ↑ William B. Breuer (2002). Deceptions of World War II. Wiley & Sons. ISBN 9780471207474.
- ↑ The California Indians, a clever satire on the government's dealings with its Indian wards. From his Crusoe's island ... 1864, p. 284-308, Publication date 1919? John Ross Browne, 1821-1875 https://archive.org/details/californiaindians00browrich/page/8/mode/2up accessed 30 March 2023
- ↑ Jeffrey D. Schultz; Luchen Li (2005). "East of Eden". Critical Companion to John Steinbeck. Infobase Publishing. p. 68. ISBN 1438108508.
- ↑ Steinbeck, John (1986). "21:4". East of Eden . Penguin Books. ISBN 978-0-14-004997-8.
- ↑ Steinbeck, John (2006) [1936]. In Dubious Battle. Introduction and notes by Warren French. New York, New York: Penguin Books. p. 99. ISBN 0-14-30-3963-6. LCCN 92-10702.
- ↑ Bernard., Cornwell (1994). Copperhead (1st ed.). New York, NY: HarperCollinsPublishers. ISBN 0060177667. OCLC 28584240.