
Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain.

Cypermethrin (CP) is a synthetic pyrethroid used as an insecticide in large-scale commercial agricultural applications as well as in consumer products for domestic purposes. It behaves as a fast-acting neurotoxin in insects. It is easily degraded on soil and plants but can be effective for weeks when applied to indoor inert surfaces. Exposure to sunlight, water and oxygen will accelerate its decomposition. Cypermethrin is highly toxic to fish, bees and aquatic insects, according to the National Pesticides Telecommunications Network (NPTN). It is found in many household ant and cockroach killers, including Raid, Ortho, Combat, ant chalk, and some products of Baygon in Southeast Asia.

Piperonyl butoxide (PBO) is a pale yellow to light brown liquid organic compound used as a synergist component of pesticide formulations. That is, despite having no pesticidal activity of its own, it enhances the potency of certain pesticides such as carbamates, pyrethrins, pyrethroids, and rotenone. It is a semisynthetic derivative of safrole.
Under United States law, pesticide misuse is considered to be the use of a pesticide in a way that violates laws regulating their use or endangers humans or the environment; many of these regulations are laid out in the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). The most common instances of pesticide misuse are applications inconsistent with the labeling, which can include the use of a material in any way not described on the label, changing dosage rates, or violating specific safety instructions. Pesticide labels have been criticized as a poor risk communication vehicle, leading some officials and researchers to question whether "misuse" is an appropriate term for what are often "unintended uses" resulting from a poor understanding of safety and application instructions. Other kinds of pesticide misuse include the sale or use of an unregistered pesticide or one whose registration has been revoked and the sale or use of an adulterated or misbranded pesticide. Under most jurisdictions, it is illegal to alter or remove pesticide labels, to sell restricted pesticides to an uncertified applicator, or to fail to maintain sales and use records of restricted pesticides.

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.

Bifenthrin is a pyrethroid insecticide. It is widely used against ant infestations.

Carbofuran is a carbamate pesticide, widely used around the world to control insects on a wide variety of field crops, including potatoes, corn and soybeans. It is a systemic insecticide, which means that the plant absorbs it through the roots, and from there the plant distributes it throughout its organs where insecticidal concentrations are attained. Carbofuran also has contact activity against pests. It is one of the most toxic pesticides still in use.

In organic chemistry, a carbamate is a category of organic compounds with the general formula R2NC(O)OR and structure >N−C(=O)−O−, which are formally derived from carbamic acid. The term includes organic compounds, formally obtained by replacing one or more of the hydrogen atoms by other organic functional groups; as well as salts with the carbamate anion H2NCOO−.

Imidacloprid is a systemic insecticide belonging to a class of chemicals called the neonicotinoids which act on the central nervous system of insects. The chemical works by interfering with the transmission of stimuli in the insect nervous system. Specifically, it causes a blockage of the nicotinergic neuronal pathway. By blocking nicotinic acetylcholine receptors, imidacloprid prevents acetylcholine from transmitting impulses between nerves, resulting in the insect's paralysis and eventual death. It is effective on contact and via stomach action. Because imidacloprid binds much more strongly to insect neuron receptors than to mammal neuron receptors, this insecticide is more toxic to insects than to mammals.
A nematicide is a type of chemical pesticide used to kill plant-parasitic nematodes. Nematicides have tended to be broad-spectrum toxicants possessing high volatility or other properties promoting migration through the soil. Aldicarb (Temik), a carbamate insecticide marketed by Bayer CropScience, is an example of a commonly used commercial nematicide. It is important in potato production, where it has been used for control of soil-borne nematodes. Aldicarb is a cholinesterase inhibitor, which prevents the breakdown of acetylcholine in the synapse. In case of severe poisoning, the victim dies of respiratory failure. It is no longer authorised for use in the EU and, in August 2010, Bayer CropScience announced that it planned to discontinue aldicarb by 2014. Human health safety and environmental concerns have resulted in the widespread deregistration of several other agronomically important nematicides. Prior to 1985, the persistent halocarbon DBCP was a widely used nematicide and soil fumigant. However, it was banned from use after being linked to sterility among male workers; the Dow Chemical company was subsequently found liable for more than $600 million in damages.
Pesticides vary in their effects on bees. Contact pesticides are usually sprayed on plants and can kill bees when they crawl over sprayed surfaces of plants or other areas around it. Systemic pesticides, on the other hand, are usually incorporated into the soil or onto seeds and move up into the stem, leaves, nectar, and pollen of plants.

Dieldrin is an organochloride originally produced in 1948 by J. Hyman & Co, Denver, as an insecticide. Dieldrin is closely related to aldrin, which reacts further to form dieldrin. Aldrin is not toxic to insects; it is oxidized in the insect to form dieldrin which is the active compound. Both dieldrin and aldrin are named after the Diels-Alder reaction which is used to form aldrin from a mixture of norbornadiene and hexachlorocyclopentadiene.

Aldicarb is a carbamate insecticide which is the active substance in the pesticide Temik. It is effective against thrips, aphids, spider mites, lygus, fleahoppers, and leafminers, but is primarily used as a nematicide. Aldicarb is a cholinesterase inhibitor which prevents the breakdown of acetylcholine in the synapse. In case of severe poisoning, the victim dies of respiratory failure.

Fipronil is a broad-spectrum insecticide that belongs to the phenylpyrazole chemical family. Fipronil disrupts the insect central nervous system by blocking the ligand-gated ion channel of the GABAA receptor and glutamate-gated chloride (GluCl) channels. This causes hyperexcitation of contaminated insects' nerves and muscles. Fipronil's specificity towards insects is believed to be due to its greater binding affinity to the GABAA receptors of insects, than to those of mammals, and to its action on GluCl channels, which do not exist in mammals. As of 2017, there did not appear to be significant resistance among fleas to fipronil.

Deltamethrin is a pyrethroid ester insecticide. Deltamethrin plays a key role in controlling malaria vectors, and is used in the manufacture of long-lasting insecticidal mosquito nets; however, resistance of mosquitos and bed bugs to deltamethrin has seen a widespread increase.

Fenoxycarb is a carbamate insect growth regulator. It has a low toxicity for bees, birds, and humans, but is toxic to fish. The oral LD50 for rats is greater than 16,800 milligrams per kilogram (0.269 oz/lb).
Neonicotinoids are a class of neuro-active insecticides chemically similar to nicotine, developed by scientists at Shell and Bayer in the 1980s.
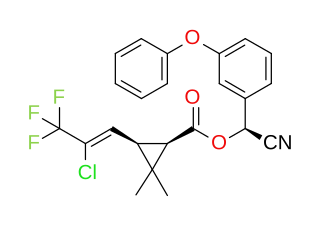
Cyhalothrin is the ISO common name for an organic compound that, in specific isomeric forms, is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as cyhalothrin are often preferred as an active ingredient in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. λ-and γ-cyhalothrin are now used to control insects and spider mites in crops including cotton, cereals, potatoes and vegetables.

Acetamiprid is an organic compound with the chemical formula C10H11ClN4. It is an odorless neonicotinoid insecticide produced under the trade names Assail, and Chipco by Aventis CropSciences. It is systemic and intended to control sucking insects (Thysanoptera, Hemiptera, mainly aphids) on crops such as leafy vegetables, citrus fruits, pome fruits, grapes, cotton, cole crops, and ornamental plants. It is also a key pesticide in commercial cherry farming due to its effectiveness against the larvae of the cherry fruit fly.

Nereistoxin is a natural product identified in 1962 as the toxic organic compound N,N-dimethyl-1,2-dithiolan-4-amine. It had first been isolated in 1934 from the marine annelid Lumbriconereis heteropoda and acts by blocking the nicotinic acetylcholine receptor. Researchers at Takeda in Japan investigated it as a possible insecticide. They subsequently developed a number of derivatives that were commercialised, including those with the ISO common names bensultap, cartap, thiocyclam and thiosultap.
















