Contents
- Production
- Uses
- Disinfectant, deodorant, and pesticide
- Precursor to other chemicals
- As ceruminolytic agent
- Environmental and health effects
- Biodegradation
- See also
- References
- External links
| |||
 1,4-dichlorobenzene crystallised on paper from DCM solution | |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 1,4-Dichlorobenzene | |||
| Other names 1,4-DCB para-Dichlorobenzene p-Dichlorobenzene p-DCB PDCB Paramoth Para crystals Paracide Dichlorocide | |||
| Identifiers | |||
3D model (JSmol) | |||
| 1680023 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.092 | ||
| EC Number |
| ||
| 49722 | |||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 3077 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C6H4Cl2 | |||
| Molar mass | 147.00 g·mol−1 | ||
| Appearance | Colorless/white crystals [1] | ||
| Odor | mothball-like [1] | ||
| Density | 1.25 g/cm3, solid | ||
| Melting point | 53.5 °C (128.3 °F; 326.6 K) | ||
| Boiling point | 174 °C (345 °F; 447 K) | ||
| 10.5 mg/100 mL (20 °C) | |||
| Vapor pressure | 1.3 mmHg (20 °C) [1] | ||
| −82.93·10−6 cm3/mol | |||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Suspected carcinogen | ||
| GHS labelling: | |||
   | |||
| Warning | |||
| H302, H315, H317, H319, H332, H335, H351, H410 | |||
| P201, P202, P261, P264, P270, P271, P272, P273, P280, P281, P301+P312, P302+P352, P304+P312, P304+P340, P305+P351+P338, P308+P313, P312, P321, P330, P332+P313, P333+P313, P337+P313, P362, P363, P391, P403+P233, P405, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 66 °C (151 °F; 339 K) | ||
| Explosive limits | 2.5%-? [1] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 500 mg/kg (rat, oral) 2950 mg/kg (mouse, oral) 2512 mg/kg (rat, oral) 2830 mg/kg (rabbit, oral) [2] | ||
LDLo (lowest published) | 857 mg/kg (human, oral) 4000 mg/kg (rat, oral) 2800 mg/kg (guinea pig, oral) [2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 75 ppm (450 mg/m3) [1] | ||
REL (Recommended) | Ca [1] | ||
IDLH (Immediate danger) | Ca [150 ppm] [1] | ||
| Related compounds | |||
Related compounds | 1,2-Dichlorobenzene 1,3-Dichlorobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
1,4-Dichlorobenzene (1,4-DCB, p-DCB, or para-dichlorobenzene, sometimes abbreviated as PDCB or para) is an aryl chloride and isomer of dichlorobenzene with the formula C6H4Cl2. This colorless solid has a strong odor. The molecule consists of a benzene ring with two chlorine atoms (replacing hydrogen atoms) on opposing sites of the ring.
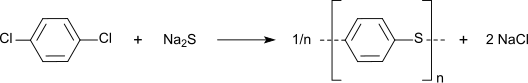
It is used as a disinfectant, pesticide, and deodorant, most familiarly in mothballs in which it is a replacement for the more traditional naphthalene because of naphthalene's greater flammability (though both chemicals have the same NFPA 704 rating). It is also used as a precursor in the production of the chemically and thermally resistant polymer poly(p-phenylene sulfide). [3]