 | |
 | |
| Names | |
|---|---|
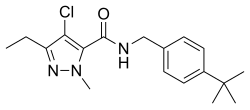
| Preferred IUPAC name N-[(4-tert-Butylphenyl)methyl]-4-chloro-3-ethyl-1-methyl-1H-pyrazole-5-carboxamide | |
| Other names 4-Chloro-N-{{#parsoidfragment:0}}4-(1,1-dimethylethyl)phenyl]]methyl]-3-ethyl-1-methyl-1H-pyrazole-5-carboxamide | |
| Identifiers | |
3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.122.745 |
| KEGG |
|
PubChem CID | |
| UNII | |
CompTox Dashboard (EPA) | |
| |
| |
| Properties | |
| C18H24ClN3O | |
| Molar mass | 333.86 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 0.5 g/mL at 24.1 °C |
| Melting point | 64 to 66 °C (147 to 151 °F; 337 to 339 K) |
| 2.61 ppm at pH 5.9 3.21 ppm at pH 4 2.39 ppm at pH 7 2.32 ppm at pH 10 | |
| Acidity (pKa) | 5.9 in water |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Tebufenpyrad is an insecticide and acaricide widely used in greenhouses. [2] It is a white solid chemical with a slight aromatic smell. It is soluble in water and also in organic solvents. [3]