
Bansal, Raj K. (2003). A Textbook of Organic Chemistry. New Age International Limited. p. 644. ISBN 978-81-224-1459-2.

Ephedra is a medicinal preparation from the plant Ephedra sinica. Several additional species belonging to the genus Ephedra have traditionally been used for a variety of medicinal purposes, and are a possible candidate for the soma plant of Indo-Iranian religion. It has been used in traditional Chinese medicine, in which it is referred to as Ma Huang, for more than 2,000 years. Native Americans and Mormon pioneers drank a tea brewed from other Ephedra species, called "Mormon tea" and "Indian tea".
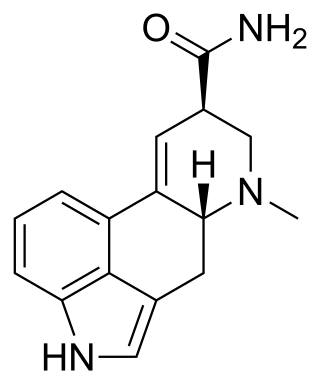
Ergine, also known as d-lysergic acid amide (LSA) and d-lysergamide, is an ergoline alkaloid that occurs in various species of vines of the Convolvulaceae and some species of fungi. The psychedelic properties in the seeds of ololiuhqui, Hawaiian baby woodrose and morning glories have been linked to ergine and/or isoergine, its epimer, as it is an alkaloid present in the seeds.
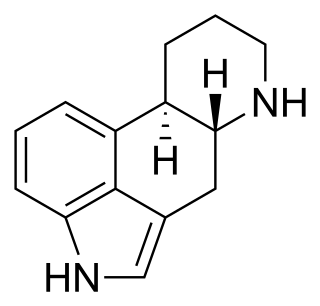
Ergoline is a chemical compound whose structural skeleton is contained in a variety of alkaloids, referred to as ergoline derivatives or ergoline alkaloids. Ergoline alkaloids, one being ergine, were initially characterized in ergot. Some of these are implicated in the condition ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives.

Harmala alkaloids are several alkaloids that increase effects of reward system neurotransmitter dopamine by acting as monoamine oxidase inhibitors (MAOIs). These alkaloids are found in the seeds of Peganum harmala, as well as leaves of tobacco and coffee beans. The alkaloids include harmine, harmaline, harmalol, and their derivatives, which have similar chemical structures, hence the name "harmala alkaloids". These alkaloids are of interest for their use in Amazonian shamanism, where they are derived from other plants. Harmine, once known as telepathine and banisterine, is a naturally occurring beta-carboline alkaloid that is structurally related to harmaline, and also found in the vine Banisteriopsis caapi. Tetrahydroharmine is also found in B. caapi and P. harmala. Dr. Alexander Shulgin has suggested that harmine may be a breakdown product of harmaline. Harmine and harmaline are reversible inhibitors of monoamine oxidase A (RIMAs). They can stimulate the central nervous system by inhibiting the metabolism of monoamine compounds such as serotonin and norepinephrine.

Harmaline is a fluorescent indole alkaloid from the group of harmala alkaloids and beta-carbolines. It is the partly hydrogenated form of harmine.

Indole alkaloids are a class of alkaloids containing a structural moiety of indole; many indole alkaloids also include isoprene groups and are thus called terpene indole or secologanin tryptamine alkaloids. Containing more than 4100 known different compounds, it is one of the largest classes of alkaloids. Many of them possess significant physiological activity and some of them are used in medicine. The amino acid tryptophan is the biochemical precursor of indole alkaloids.
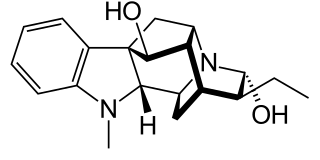
Ajmaline is an alkaloid that is classified as a 1-A antiarrhythmic agent. It is often used to induce arrhythmic contraction in patients suspected of having Brugada syndrome. Individuals suffering from Brugada syndrome will be more susceptible to the arrhythmogenic effects of the drug, and this can be observed on an electrocardiogram as an ST elevation.
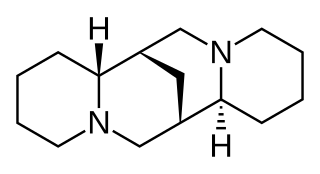
Sparteine is a class 1a antiarrhythmic agent; a sodium channel blocker. It is an alkaloid and can be extracted from scotch broom. It is the predominant alkaloid in Lupinus mutabilis, and is thought to chelate the bivalent metals calcium and magnesium. It is not FDA approved for human use as an antiarrhythmic agent, and it is not included in the Vaughan Williams classification of antiarrhythmic drugs.

Laudanosine or N-methyltetrahydropapaverine is a recognized metabolite of atracurium and cisatracurium. Laudanosine decreases the seizure threshold, and thus it can induce seizures if present at sufficient threshold concentrations; however such concentrations are unlikely to be produced consequent to chemodegradable metabolism of clinically administered doses of cisatracurium or atracurium.

Yuremamine is a phytoindole alkaloid which was isolated from the bark of Mimosa tenuiflora in 2005, and erroneously assigned a pyrrolo[1,2-a]indole structure that was thought to represent a new class of indole alkaloids. However, in 2015, the bioinspired total synthesis of yuremamine revealed its structure to be a flavonoid derivative. It was also noted in the original isolation of yuremamine that the alkaloid occurs naturally as a purple solid, but total synthesis revealed that yuremamine as a free base is colorless, and the formation of a trifluoroacetate salt during HPLC purification is what led to the purple appearance.

Akuammine (vincamajoridine) is an indole alkaloid. It is the most abundant alkaloid found in the seeds from the tree Picralima nitida, commonly known as akuamma, comprising 0.56% of the dried powder. It has also been isolated from Vinca major. Akuammine is structurally related to yohimbine, mitragynine and more distantly Voacangine, all of which are alkaloid plant products with pharmacological properties.
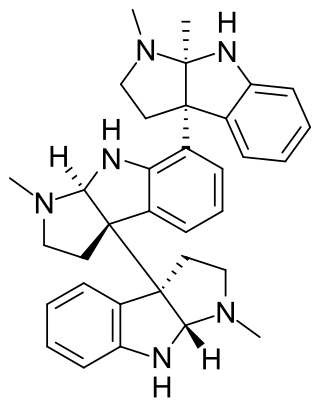
Hodgkinsine is an alkaloid found in plants of the genus Psychotria, particularly Psychotria colorata, although it is also found in Psychotria lyciiflora and probably other species in this family,

Conessine is a steroidal alkaloid found in a number of plant species from the family Apocynaceae, including Holarrhena floribunda, Holarrhena antidysenterica and Funtumia elastica. It acts as a histamine antagonist, selective for the H3 subtype (with an affinity of pKi = 8.27; Ki = ~5 nM). It was also found to have long CNS clearance times, high blood–brain barrier penetration and high affinity for the adrenergic receptors.

Toxiferine is a curare toxin. It is a bisindole alkaloid derived from Strychnos toxifera and a nicotinic acetylcholine receptor antagonist. This alkaloid is the main toxic component of Calabash curare, and one of the most toxic plant alkaloids known. The lethal dose (LD50) for mice has been determined as 10 - 60 µg/kg by intravenous administration. It is a muscle relaxant that causes paralysis of skeletal muscle, which takes approximately 2 hours to recovery for a moderate dose, and 8 hours of total paralysis with a 20-fold paralytic dose. The paralysis can be antagonized by neostigmine
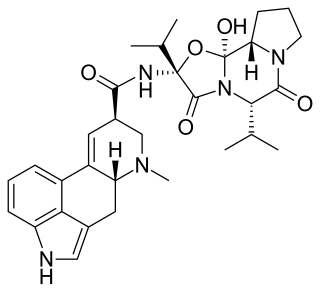
Ergocornine is a crystalline ergopeptine and one of the ergot alkaloids separated from ergotoxine. It is also a dopamine receptor agonist. It was discovered by Albert Hofmann, the Swiss chemist who created LSD.

Coronaridine, also known as 18-carbomethoxyibogamine, is an alkaloid found in Tabernanthe iboga and related species, including Tabernaemontana divaricata for which it was named.

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.
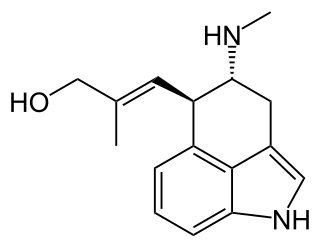
Chanoclavine, also known as chanoclavin-l is a tri-cyclic ergot alkaloid (ergoline) isolate of certain fungi. It is mainly produced by members of the genus claviceps. Long used in traditional Chinese medicine, it was found in 1987 mouse studies to stimulate dopamine D2 receptors in the brain.

Apparicine is a monoterpenoid indole alkaloid. It is named after Apparicio Duarte, a Brazilian botanist who studied the Aspidosperma species from which apparicine was first isolated. It was the first member of the vallesamine group of alkaloids to be isolated and have its structure established, which was first published in 1965. It has also been known by the synonyms gomezine, pericalline, and tabernoschizine.




















