Insecticides are pesticides used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. The major use of insecticides is in agriculture, but they are also used in home and garden settings, industrial buildings, for vector control, and control of insect parasites of animals and humans.
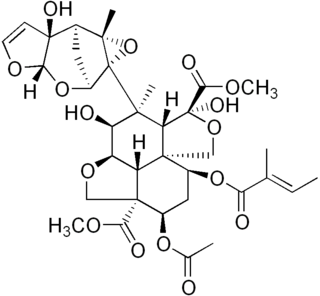
Azadirachtin, a chemical compound belonging to the limonoid group, is a secondary metabolite present in neem seeds. It is a highly oxidized tetranortriterpenoid which boasts a plethora of oxygen-bearing functional groups, including an enol ether, acetal, hemiacetal, tetra-substituted epoxide and a variety of carboxylic esters.

The pyrethrins are a class of organic compounds normally derived from Chrysanthemum cinerariifolium that have potent insecticidal activity by targeting the nervous systems of insects. Pyrethrin naturally occurs in chrysanthemum flowers and is often considered an organic insecticide when it is not combined with piperonyl butoxide or other synthetic adjuvants. Their insecticidal and insect-repellent properties have been known and used for thousands of years.
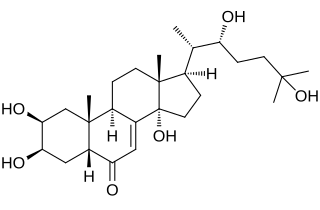
Ecdysone is a prohormone of the major insect molting hormone 20-hydroxyecdysone, secreted from the prothoracic glands. It is of steroidal structure. Insect molting hormones are generally called ecdysteroids. Ecdysteroids act as moulting hormones of arthropods but also occur in other related phyla where they can play different roles. In Drosophila melanogaster, an increase in ecdysone concentration induces the expression of genes coding for proteins that the larva requires. It causes chromosome puffs to form in polytene chromosomes. Recent findings in the laboratory of Chris Q. Doe have found a novel role of this hormone in regulating temporal gene transitions within neural stem cells of the fruit fly.

Imidacloprid is a systemic insecticide belonging to a class of chemicals called the neonicotinoids which act on the central nervous system of insects. The chemical works by interfering with the transmission of stimuli in the insect nervous system. Specifically, it causes a blockage of the nicotinergic neuronal pathway. By blocking nicotinic acetylcholine receptors, imidacloprid prevents acetylcholine from transmitting impulses between nerves, resulting in the insect's paralysis and eventual death. It is effective on contact and via stomach action. Because imidacloprid binds much more strongly to insect neuron receptors than to mammal neuron receptors, this insecticide is more toxic to insects than to mammals.

Fenthion is an organothiophosphate insecticide, avicide, and acaricide. Like most other organophosphates, its mode of action is via cholinesterase inhibition. Due to its relatively low toxicity towards humans and mammals, fenthion is listed as moderately toxic compound in U.S. Environmental Protection Agency and World Health Organization toxicity class.

Fenoxycarb is a carbamate insect growth regulator. It has a low toxicity for bees, birds, and humans, but is toxic to fish. The oral LD50 for rats is greater than 16,800 milligrams per kilogram (0.269 oz/lb).
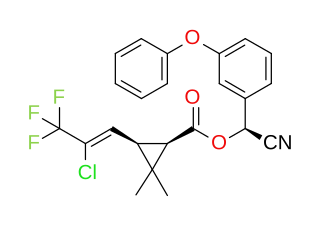
Cyhalothrin is an organic compound that, in specific isomeric forms, is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids, such as cyhalothrin, are often preferred as an active ingredient in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. λ-and γ-cyhalothrin are now used to control insects and spider mites in crops including cotton, cereals, potatoes and vegetables.
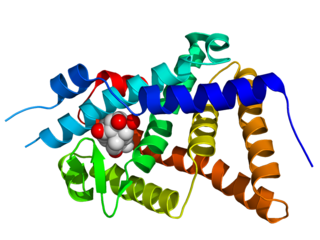
The ecdysone receptor is a nuclear receptor found in arthropods, where it controls development and contributes to other processes such as reproduction. The receptor is a non-covalent heterodimer of two proteins, the EcR protein and ultraspiracle protein (USP). It binds to and is activated by ecdysteroids. Insect ecdysone receptors are currently better characterized than those from other arthropods, and mimics of ecdysteroids are used commercially as caterpillar-selective insecticides.

Acetamiprid is an organic compound with the chemical formula C10H11ClN4. It is an odorless neonicotinoid insecticide produced under the trade names Assail, and Chipco by Aventis CropSciences. It is systemic and intended to control sucking insects (Thysanoptera, Hemiptera, mainly aphids) on crops such as leafy vegetables, citrus fruits, pome fruits, grapes, cotton, cole crops, and ornamental plants. It is also a key pesticide in commercial cherry farming due to its effectiveness against the larvae of the cherry fruit fly.

The southwestern corn borer, Diatraea grandiosella, is a moth belonging to the sub-order Heterocera. Like most moths, The southwestern corn borer undergoes complete metamorphosis developing as an egg, larva (caterpillar), pupa and adult. It is capable of entering diapause in its larva stage and under the conditions of a precise photoperiod. Growth and development are regulated by juvenile hormones. The southwestern corn borer has an extensive range. It occurs in Mexico and in Alabama, Arizona, Arkansas, Colorado, Illinois, Indiana, Kansas, Kentucky, Louisiana, Mississippi, Missouri, Nebraska, New Mexico, Oklahoma, Tennessee, and Texas.
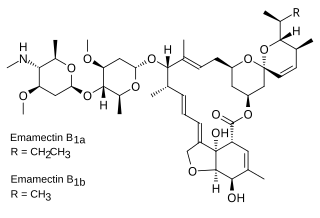
Emamectin is the 4″-deoxy-4″-methylamino derivative of abamectin, a 16-membered macrocyclic lactone produced by the fermentation of the soil actinomycete Streptomyces avermitilis. It is generally prepared as the salt with benzoic acid, emamectin benzoate, which is a white or faintly yellow powder. Emamectin is widely used in the US and Canada as an insecticide because of its chloride channel activation properties.
An insect growth regulator (IGR) is a chemical insecticide that kills insects indirectly by disrupting their life cycles. The term was initially proposed to describe the effects of juvenile hormone analogs. Although the term "insect growth disruptor" more accurately describes the actions of IGRs, it did not become widely used. IGRs encompass chemical classes with three modes of action : juvenile hormone analogs, chitin synthesis inhibitors, and ecdysone receptor agonists.

Diflubenzuron is an insecticide of the benzoylurea class. It is used in forest management and on field crops to selectively control insect pests, particularly forest tent caterpillar moths, boll weevils, gypsy moths, and other types of moths. It is a widely used larvicide in India for control of mosquito larvae by public health authorities. Diflubenzuron is approved by the WHO Pesticide Evaluation Scheme.
Lymantria dispar multicapsid nuclear polyhedrosis virus or LdMNPV is a viral infection in spongy moths that causes infected larvae to die and disintegrate. Infected larvae climb to the top of a tree and die. The larvae then melt or disintegrate, falling onto the foliage below, where they infect more larvae.

Diamide insecticides are a class of insecticides, active mainly against lepidoptera (caterpillars), which act on the insect ryanodine receptor. They are diamides of either phthalic acid or anthranilic acid, with various appropriate further substitutions.
Chilo infuscatellus, the yellow top borer or sugarcane shoot borer, is a moth in the family Crambidae. It was described by the Dutch entomologist Samuel Constantinus Snellen van Vollenhoven in 1890. It is found in India, Myanmar, Tajikistan, Afghanistan, Korea, Taiwan, Malaysia, the Philippines and on Java and Timor.
Trichogramma japonicum is a minute wasp parasitoid from the Trichogrammatidae family in the order Hymenoptera. T. japonicum parasitizes the eggs of many pest species, especially Lepidoptera found in many monocultures. They are entomophagous parasitoids that deposit their eggs inside the host species' egg, consuming the host egg material and emerging from the egg once development is complete. T. japonicum can be found naturally in rice ecosystems, but are dispersed commercially to many monocultures as a biological control. The mitochondrial genomes of T. japonicum are significantly rearranged when comparing it to related insects.
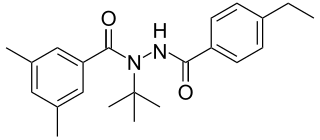
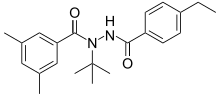
The diacylhydrazines, also known as bisacylhydrazines (BAHs) or dibenzoylhydrazines are appropriately substituted derivatives of dibenzoyl hydrazine. They do not immediately kill the insect, but produces premature unsuccessful moulting, which then causes the death of the insect. BAHs thus belong to the class of insect growth regulators.