Inositol trisphosphate or inositol 1,4,5-trisphosphate abbreviated InsP3 or Ins3P or IP3 is an inositol phosphate signaling molecule. It is made by hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2), a phospholipid that is located in the plasma membrane, by phospholipase C (PLC). Together with diacylglycerol (DAG), IP3 is a second messenger molecule used in signal transduction in biological cells. While DAG stays inside the membrane, IP3 is soluble and diffuses through the cell, where it binds to its receptor, which is a calcium channel located in the endoplasmic reticulum. When IP3 binds its receptor, calcium is released into the cytosol, thereby activating various calcium regulated intracellular signals.

The sodium–potassium pump is an enzyme found in the membrane of all animal cells. It performs several functions in cell physiology.
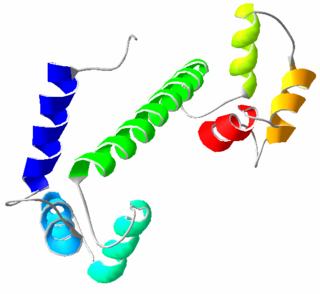
Calmodulin (CaM) (an abbreviation for calcium-modulated protein) is a multifunctional intermediate calcium-binding messenger protein expressed in all eukaryotic cells. It is an intracellular target of the secondary messenger Ca2+, and the binding of Ca2+ is required for the activation of calmodulin. Once bound to Ca2+, calmodulin acts as part of a calcium signal transduction pathway by modifying its interactions with various target proteins such as kinases or phosphatases.

The adrenergic receptors or adrenoceptors are a class of G protein-coupled receptors that are targets of many catecholamines like norepinephrine (noradrenaline) and epinephrine (adrenaline) produced by the body, but also many medications like beta blockers, beta-2 (β2) agonists and alpha-2 (α2) agonists, which are used to treat high blood pressure and asthma, for example.
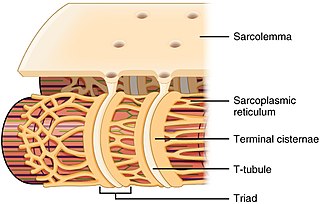
The sarcoplasmic reticulum (SR) is a membrane-bound structure found within muscle cells that is similar to the smooth endoplasmic reticulum in other cells. The main function of the SR is to store calcium ions (Ca2+). Calcium ion levels are kept relatively constant, with the concentration of calcium ions within a cell being 10,000 times smaller than the concentration of calcium ions outside the cell. This means that small increases in calcium ions within the cell are easily detected and can bring about important cellular changes (the calcium is said to be a second messenger). Calcium is used to make calcium carbonate (found in chalk) and calcium phosphate, two compounds that the body uses to make teeth and bones. This means that too much calcium within the cells can lead to hardening (calcification) of certain intracellular structures, including the mitochondria, leading to cell death. Therefore, it is vital that calcium ion levels are controlled tightly, and can be released into the cell when necessary and then removed from the cell.
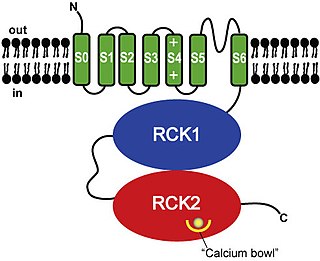
BK channels (big potassium), are large conductance calcium-activated potassium channels, also known as Maxi-K, slo1, or Kca1.1. BK channels are voltage-gated potassium channels that conduct large amounts of potassium ions (K+) across the cell membrane, hence their name, big potassium. These channels can be activated (opened) by either electrical means, or by increasing Ca2+ concentrations in the cell. BK channels help regulate physiological processes, such as circadian behavioral rhythms and neuronal excitability. BK channels are also involved in many processes in the body, as it is a ubiquitous channel. They have a tetrameric structure that is composed of a transmembrane domain, voltage sensing domain, potassium channel domain, and a cytoplasmic C-terminal domain, with many X-ray structures for reference. Their function is to repolarize the membrane potential by allowing for potassium to flow outward, in response to a depolarization or increase in calcium levels.
Voltage-gated calcium channels (VGCCs), also known as voltage-dependent calcium channels (VDCCs), are a group of voltage-gated ion channels found in the membrane of excitable cells (e.g. muscle, glial cells, neurons) with a permeability to the calcium ion Ca2+. These channels are slightly permeable to sodium ions, so they are also called Ca2+–Na+ channels, but their permeability to calcium is about 1000-fold greater than to sodium under normal physiological conditions.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
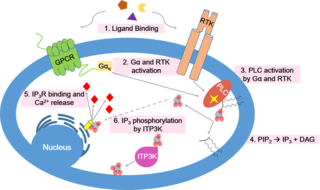
Calcium signaling is the use of calcium ions (Ca2+) to communicate and drive intracellular processes often as a step in signal transduction. Ca2+ is important for cellular signalling, for once it enters the cytosol of the cytoplasm it exerts allosteric regulatory effects on many enzymes and proteins. Ca2+ can act in signal transduction resulting from activation of ion channels or as a second messenger caused by indirect signal transduction pathways such as G protein-coupled receptors.
There are at least four known endothelin receptors, ETA, ETB1, ETB2 and ETC, all of which are G protein-coupled receptors whose activation result in elevation of intracellular-free calcium, which constricts the smooth muscles of the blood vessels, raising blood pressure, or relaxes the smooth muscles of the blood vessels, lowering blood pressure, among other functions.

The inorganic dye ammoniated ruthenium oxychloride, also known as ruthenium red, is used in histology to stain aldehyde fixed mucopolysaccharides.

Cyclic ADP-ribose, frequently abbreviated as cADPR, is a cyclic adenine nucleotide (like cAMP) with two phosphate groups present on 5' OH of the adenosine (like ADP), further connected to another ribose at the 5' position, which, in turn, closes the cycle by glycosidic bonding to the nitrogen 1 (N1) of the same adenine base (whose position N9 has the glycosidic bond to the other ribose). The N1-glycosidic bond to adenine is what distinguishes cADPR from ADP-ribose (ADPR), the non-cyclic analog. cADPR is produced from nicotinamide adenine dinucleotide (NAD+) by ADP-ribosyl cyclases (EC 3.2.2.5) as part of a second messenger system.

Translocator protein (TSPO) is an 18 kDa protein mainly found on the outer mitochondrial membrane. It was first described as peripheral benzodiazepine receptor (PBR), a secondary binding site for diazepam, but subsequent research has found the receptor to be expressed throughout the body and brain. In humans, the translocator protein is encoded by the TSPO gene. It belongs to a family of tryptophan-rich sensory proteins. Regarding intramitochondrial cholesterol transport, TSPO has been proposed to interact with StAR to transport cholesterol into mitochondria, though evidence is mixed.

The calcium-sensing receptor (CaSR) is a Class C G-protein coupled receptor which senses extracellular levels of calcium ions. It is primarily expressed in the parathyroid gland, the renal tubules of the kidney and the brain. In the parathyroid gland, it controls calcium homeostasis by regulating the release of parathyroid hormone (PTH). In the kidney it has an inhibitory effect on the reabsorption of calcium, potassium, sodium, and water depending on which segment of the tubule is being activated.

Ryanodine receptor 2 (RYR2) is one of a class of ryanodine receptors and a protein found primarily in cardiac muscle. In humans, it is encoded by the RYR2 gene. In the process of cardiac calcium-induced calcium release, RYR2 is the major mediator for sarcoplasmic release of stored calcium ions.

Ryanodine receptor 1 (RYR-1) also known as skeletal muscle calcium release channel or skeletal muscle-type ryanodine receptor is one of a class of ryanodine receptors and a protein found primarily in skeletal muscle. In humans, it is encoded by the RYR1 gene.

Ryanodine receptor 3 is one of a class of ryanodine receptors and a protein that in humans is encoded by the RYR3 gene. The protein encoded by this gene is both a calcium channel and a receptor for the plant alkaloid ryanodine. RYR3 and RYR1 control the resting calcium ion concentration in skeletal muscle.
Imperatoxin I (IpTx) is a peptide toxin derived from the venom of the African scorpion Pandinus imperator.
The ryanodine-inositol 1,4,5-triphosphate receptor Ca2+ channel (RIR-CaC) family includes Ryanodine receptors and Inositol trisphosphate receptors. Members of this family are large proteins, some exceeding 5000 amino acyl residues in length. This family belongs to the Voltage-gated ion channel (VIC) superfamily. Ry receptors occur primarily in muscle cell sarcoplasmic reticular (SR) membranes, and IP3 receptors occur primarily in brain cell endoplasmic reticular (ER) membranes where they effect release of Ca2+ into the cytoplasm upon activation (opening) of the channel. They are redox sensors, possibly providing a partial explanation for how they control cytoplasmic Ca2+. Ry receptors have been identified in heart mitochondria where they provide the main pathway for Ca2+ entry. Sun et al. (2011) have demonstrated oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor-Ca2+ release channel (RyR1;TC# 1.A.3.1.2) by NADPH oxidase 4.
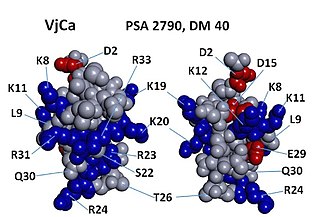
Vejocalcin is a toxin from the venom of the Mexican scorpion Vaejovis mexicanus. Vejocalcin is a member of the calcin family of toxins. It acts as a cell-penetrating peptide (CPP); it binds with high affinity and specificity to skeletal ryanodine receptor 1 (RYR1) of the sarcoplasmic reticulum, thereby triggering calcium release from intracellular Ca2+
stores.














