
Paraquat (trivial name; ), or N,N′-dimethyl-4,4′-bipyridinium dichloride (systematic name), also known as methyl viologen, is an organic compound with the chemical formula [(C6H7N)2]Cl2. It is classified as a viologen, a family of redox-active heterocycles of similar structure. This salt is one of the most widely used herbicides. It is quick-acting and non-selective, killing green plant tissue on contact. It is also toxic (lethal) to human beings and animals due to its redox activity, which produces superoxide anions. It has been linked to the development of Parkinson's disease and is banned in 58 countries.

VG is a "V-series" nerve agent chemically similar to the better-known VX nerve agent. Tetram is the common Russian name for the substance. Amiton was the trade name for the substance when it was marketed as an insecticide by ICI in the mid-1950s.
Demeton-S-methyl is an organic compound with the molecular formula C6H15O3PS2. It was used as an organothiophosphate acaricide and organothiophosphate insecticide. It is flammable. With prolonged storage, Demeton-S-methyl becomes more toxic due to formation of a sulfonium derivative which has greater affinity to the human form of the acetylcholinesterase enzyme, and this may present a hazard in agricultural use.
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups.

Diquat is the ISO common name for an organic dication that, as a salt with counterions such as bromide or chloride is used as a contact herbicide that produces desiccation and defoliation. Diquat is no longer approved for use in the European Union, although its registration in many other countries including the USA is still valid.

Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of carbamic acid (H2NCO2H). It is a colourless solid.

Acibenzolar-S-methyl is the ISO common name for an organic compound that is used as a fungicide. Unusually, it is not directly toxic to fungi but works by inducing systemic acquired resistance, the natural defence system of plants.
A seed treatment is a treatment of the seed with either biological or chemical agents or by physical methods. Usually done to provide protection to the seed and improve the establishment of healthy crops. Not to be confused with a seed coating.

Azoxystrobin is a broad spectrum systemic fungicide widely used in agriculture to protect crops from fungal diseases. It was first marketed in 1996 using the brand name Amistar and by 1999 it had been registered in 48 countries on more than 50 crops. In the year 2000 it was announced that it had been granted UK Millennium product status.
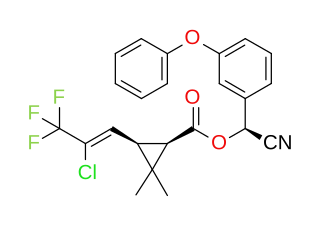
Cyhalothrin is the ISO common name for an organic compound that, in specific isomeric forms, is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as cyhalothrin are often preferred as an active ingredient in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. λ-and γ-cyhalothrin are now used to control insects and spider mites in crops including cotton, cereals, potatoes and vegetables.

Indoxacarb is an oxadiazine pesticide developed by DuPont that acts against lepidopteran larvae. It is marketed under the names Indoxacarb Technical Insecticide, Steward Insecticide and Avaunt Insecticide. It is also used as the active ingredient in the Syngenta line of commercial pesticides: Advion and Arilon.
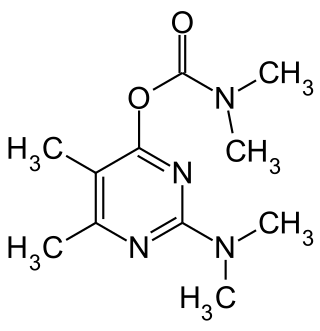
Pirimicarb is a selective carbamate insecticide used to control aphids on vegetable, cereal and orchard crops by inhibiting acetylcholinesterase activity but does not affect useful predators such as ladybirds that eat them. It was originally developed by Imperial Chemical Industries Ltd., now Syngenta, at their Jealott's Hill site and first marketed in 1969, four years after its discovery.
Jealott's Hill is a village in the county of Berkshire, England, within the civil parish of Warfield. The settlement is on the A3095 road approximately 3 miles (5 km) north of Bracknell. The nearest railway station is in Bracknell. The name of the hill is reported to have derived from the surname of a 14th-century landowner, Roger Jolyl. This name evolved into "Joyliff's Hill" and then, on Henry Walter's Map of Windsor Forest, 1823, became "Jealous Hill". This changed again to "Jealot's Hill" on John Snare's 1846 map and by the 1920s the modern spelling was established.

Thiamethoxam is the ISO common name for a mixture of cis-trans isomers used as a systemic insecticide of the neonicotinoid class. It has a broad spectrum of activity against many types of insects and can be used as a seed dressing.

Tefluthrin is the ISO common name for an organic compound that is used as a pesticide. It is a pyrethroid, a class of synthetic insecticides that mimic the structure and properties of the naturally occurring insecticide pyrethrin which is present in the flowers of Chrysanthemum cinerariifolium. Pyrethroids such as tefluthrin are often preferred as active ingredients in agricultural insecticides because they are more cost-effective and longer acting than natural pyrethrins. It is effective against soil pests because it can move as a vapour without irreversibly binding to soil particles: in this respect it differs from most other pyrethroids.
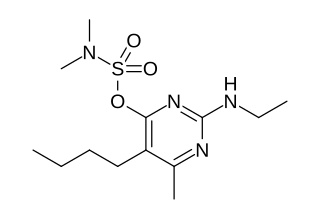
Bupirimate is an active ingredient of plant protection products, which has an effect as a fungicide. It belongs to the chemical family of pyrimidine sulfamates. Bupirimate has translaminar mobility and systemic translocation in the xylem. It acts mainly by inhibiting sporulation and is used for control of powdery mildew of apples, pears, stone fruit, cucurbits, roses and other ornamentals, strawberries, gooseberries, currants, raspberries, hops, beets and other crops. Bupirimate is not an insecticide. It is of low mammalian toxicity and is non-toxic to bees. However, it is used in many products which also contain insecticides.

Parathion methyl, or methyl parathion, is an organophosphate insecticide, possessing an organothiophosphate group. It is structurally very similar to parathion-ethyl. It is not allowed for sale and import in nearly all countries around the world, while a few allow it under subject to specified conditions only.

The Fernhurst Research Station was a crop protection chemical research institute in West Sussex, mainly run by ICI, for the fruit industry. The site is to the east of the A286, around a mile south of the village of Fernhurst and a mile north of the Haslemere to Petersfield Serpent Trail.

Fomesafen is the ISO common name for an organic compound used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase (PPO) which is necessary for chlorophyll synthesis. Soybeans naturally have a high tolerance to fomesafen, via metabolic disposal by glutathione S-transferase. As a result, soy is the most common crop treated with fomesafen, followed by other beans and a few other crop types. It is not safe for maize/corn or other Poaceae.

Butafenacil is the ISO common name for an organic compound of the pyrimidinedione chemical class used as an herbicide. It acts by inhibiting the enzyme protoporphyrinogen oxidase to control broadleaf and some grass weeds in crops including cereals and canola.



















