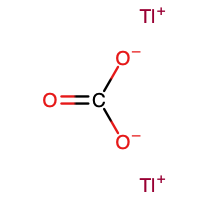Soman is an extremely toxic chemical substance. It is a nerve agent, interfering with normal functioning of the mammalian nervous system by inhibiting the enzyme cholinesterase. It is an inhibitor of both acetylcholinesterase and butyrylcholinesterase. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations according to UN Resolution 687. Its production is strictly controlled, and stockpiling is outlawed by the Chemical Weapons Convention of 1993 where it is classified as a Schedule 1 substance. Soman was the third of the so-called G-series nerve agents to be discovered along with GA (tabun), GB (sarin), and GF (cyclosarin).
A period 4 element is one of the chemical elements in the fourth row of the periodic table of the chemical elements. The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall into the same vertical columns. The fourth period contains 18 elements beginning with potassium and ending with krypton – one element for each of the eighteen groups. It sees the first appearance of d-block in the table.
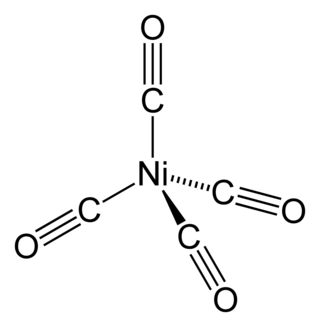
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal carbonyl of nickel. It is an intermediate in the Mond process for producing very high-purity nickel and a reagent in organometallic chemistry, although the Mond Process has fallen out of common usage due to the health hazards in working with the compound. Nickel carbonyl is one of the most dangerous substances yet encountered in nickel chemistry due to its very high toxicity, compounded with high volatility and rapid skin absorption.

Chlorfenvinphos is an organophosphorus compound that was widely used as an insecticide and an acaricide. The molecule itself can be described as an enol ester derived from dichloroacetophenone and diethylphosphonic acid. Chlorfenvinphos has been included in many products since its first use in 1963. However, because of its toxic effect as a cholinesterase inhibitor it has been banned in several countries, including the United States and the European Union. Its use in the United States was discontinued in 1991.

VG is a "V-series" nerve agent chemically similar to the better-known VX nerve agent. Tetram is the common Russian name for the substance. Amiton was the trade name for the substance when it was marketed as an insecticide by ICI in the mid-1950s.
Organochlorine chemistry is concerned with the properties of organochlorine compounds, or organochlorides, organic compounds containing at least one covalently bonded atom of chlorine. The chloroalkane class includes common examples. The wide structural variety and divergent chemical properties of organochlorides lead to a broad range of names, applications, and properties. Organochlorine compounds have wide use in many applications, though some are of profound environmental concern, with TCDD being one of the most notorious.
Cyanogen iodide or iodine cyanide (ICN) is a pseudohalogen composed of iodine and the cyanide group. It is a highly toxic inorganic compound. It occurs as white crystals that react slowly with water to form hydrogen cyanide.

The Emergency Planning and Community Right-to-Know Act of 1986 is a United States federal law passed by the 99th United States Congress located at Title 42, Chapter 116 of the U.S. Code, concerned with emergency response preparedness.

Hydrogen selenide is an inorganic compound with the formula H2Se. This hydrogen chalcogenide is the simplest and most commonly encountered hydride of selenium. H2Se is a colorless, flammable gas under standard conditions. It is the most toxic selenium compound with an exposure limit of 0.05 ppm over an 8-hour period. Even at extremely low concentrations, this compound has a very irritating smell resembling that of decayed horseradish or "leaking gas", but smells of rotten eggs at higher concentrations.
Arsenic pentoxide is the inorganic compound with the formula As2O5. This glassy, white, deliquescent solid is relatively unstable, consistent with the rarity of the As(V) oxidation state. More common, and far more important commercially, is arsenic(III) oxide (As2O3). All inorganic arsenic compounds are highly toxic and thus find only limited commercial applications.

2-Chloro-N,N-bis(2-chloroethyl)ethanamine, also known as trichlormethine, tris(2-chloroethyl)amine is the organic compound with the formula N(CH2CH2Cl)3. Often abbreviated HN3 or HN-3, it is a powerful blister agent and a nitrogen mustard used for chemical warfare. HN3 was the last of the nitrogen mustard agents developed. It was designed as a military agent and is the only one of the nitrogen mustards that is still used for military purposes. It is the principal representative of the nitrogen mustards because its vesicant properties are almost equal to those of HD and thus the analogy between the two types of mustard is the strongest. As a vesicant the use and production is strongly restricted within the Chemical Weapons Convention where it is classified as a Schedule 1 substance.
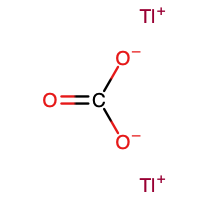
Thallium(I) carbonate is the inorganic compound with the formula Tl2CO3. It is a white, water-soluble salt. It has no or very few commercial applications. It is produced by treatment of thallous hydroxide with CO2.
Acetone cyanohydrin (ACH) is an organic compound used in the production of methyl methacrylate, the monomer of the transparent plastic polymethyl methacrylate (PMMA), also known as acrylic. It liberates hydrogen cyanide easily, so it is used as a source of such. For this reason, this cyanohydrin is also highly toxic.

Diphenadione is a vitamin K antagonist that has anticoagulant effects and is used as a rodenticide against rats, mice, voles, ground squirrels and other rodents. The chemical compound is an anti-coagulant with active half-life longer than warfarin and other synthetic 1,3-indandione anticoagulants.

Carbophenothion also known as Stauffer R 1303 as for the manufacturer, Stauffer Chemical, is an organophosphorus chemical compound. It was used as a pesticide for citrus fruits under the name of Trithion. Carbophenothion was used as an insecticide and acaricide. Although not used anymore it is still a restricted use pesticide in the United States. The chemical is identified in the US as an extremely hazardous substance according to the Emergency Planning and Community Right-to-Know Act.

The Toxin and Toxin-Target Database (T3DB), also known as the Toxic Exposome Database, is a freely accessible online database of common substances that are toxic to humans, along with their protein, DNA or organ targets. The database currently houses nearly 3,700 toxic compounds or poisons described by nearly 42,000 synonyms. This list includes various groups of toxins, including common pollutants, pesticides, drugs, food toxins, household and industrial/workplace toxins, cigarette toxins, and uremic toxins. These toxic substances are linked to 2,086 corresponding protein/DNA target records. In total there are 42,433 toxic substance-toxin target associations. Each toxic compound record (ToxCard) in T3DB contains nearly 100 data fields and holds information such as chemical properties and descriptors, mechanisms of action, toxicity or lethal dose values, molecular and cellular interactions, medical information, NMR an MS spectra, and up- and down-regulated genes. This information has been extracted from over 18,000 sources, which include other databases, government documents, books, and scientific literature.
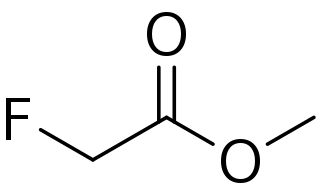
Methyl fluoroacetate (MFA) is an organic compound with the chemical formula FCH2CO2CH3. It is the extremely toxic methyl ester of fluoroacetic acid. It is a colorless, odorless liquid at room temperature. It is used as a laboratory chemical and as a rodenticide. Because of its extreme toxicity, MFA was studied for potential use as a chemical weapon.

Methylfluorophosphonylcholine (MFPCh) is an extremely toxic chemical compound related to the G-series nerve agents. It is an extremely potent acetylcholinesterase inhibitor which is around 100 times more potent than sarin at inhibiting acetylcholinesterase in vitro, and around 10 times more potent in vivo, depending on route of administration and animal species tested. MFPCh is resistant to oxime reactivators, meaning the acetylcholinesterase inhibited by MFPCh can't be reactivated by cholinesterase reactivators. MFPCh also acts directly on the acetylcholine receptors. MFPCh is a relatively unstable compound and degrades rapidly in storage, so despite its enhanced toxicity it was not deemed suitable to be weaponised for military use.

The CompTox Chemicals Dashboard is a freely accessible online database created and maintained by the U.S. Environmental Protection Agency (EPA). The database provides access to multiple types of data including physicochemical properties, environmental fate and transport, exposure, usage, in vivo toxicity, and in vitro bioassay. EPA and other scientists use the data and models contained within the dashboard to help identify chemicals that require further testing and reduce the use of animals in chemical testing. The Dashboard is also used to provide public access to information from EPA Action Plans, e.g. around perfluorinated alkylated substances.
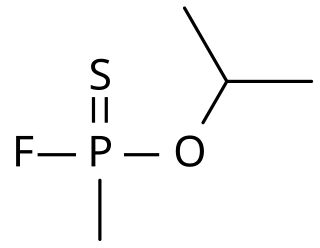
Thiosarin, sulfursarin or GBS, is the organophosphorus compound analogous to sarin. It differs structurally in that sulfur replaces the oxygen of the P=O bond. It is an extremely toxic substance related to G-agents.