Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white insoluble solid. It occurs as the mineral fluorite (also called fluorspar), which is often deeply coloured owing to impurities.
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms and no atoms of elements from any other group.
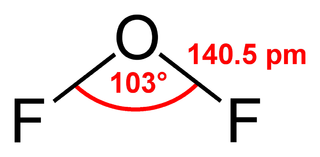
Oxygen difluoride is a chemical compound with the formula OF2. As predicted by VSEPR theory, the molecule adopts a "bent" molecular geometry similar to that of water. However, it has very different properties, being a strong oxidizer.
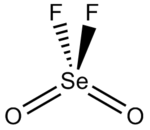
Selenic acid is the inorganic compound with the formula H2SeO4. It is an oxoacid of selenium, and its structure is more accurately described as (HO)2SeO2. It is a colorless compound. Although it has few uses, its derivative sodium selenate is used in the production of glass and animal feeds.
An inorganic nonaqueous solvent is a solvent other than water, that is not an organic compound. These solvents are used in chemical research and industry for reactions that cannot occur in aqueous solutions or require a special environment. Inorganic nonaqueous solvents can be classified into two groups, protic solvents and aprotic solvents. Early studies on inorganic nonaqueous solvents evaluated ammonia, hydrogen fluoride, sulfuric acid, as well as more specialized solvents, hydrazine, and selenium oxychloride.

Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF
2, and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive. It decomposes on contact with water vapor, but is otherwise stable in storage. Xenon difluoride is a dense, colourless crystalline solid.

Selenium tetrafluoride (SeF4) is an inorganic compound. It is a colourless liquid that reacts readily with water. It can be used as a fluorinating reagent in organic syntheses (fluorination of alcohols, carboxylic acids or carbonyl compounds) and has advantages over sulfur tetrafluoride in that milder conditions can be employed and it is a liquid rather than a gas.

Xenon oxytetrafluoride is an inorganic chemical compound. It is a colorless stable liquid with a melting point of −46.2 °C that can be synthesized by partial hydrolysis of XeF
6, or the reaction of XeF
6 with silica or NaNO
3:
Selenium trioxide is the inorganic compound with the formula SeO3. It is white, hygroscopic solid. It is also an oxidizing agent and a Lewis acid. It is of academic interest as a precursor to Se(VI) compounds.

Selenium tetrachloride is the inorganic compound composed with the formula SeCl4. This compound exists as yellow to white volatile solid. It is one of two commonly available selenium chlorides, the other example being selenium monochloride, Se2Cl2. SeCl4 is used in the synthesis of other selenium compounds.
Dinitrogen difluoride is a chemical compound with the formula N2F2. It is a gas at room temperature, and was first identified in 1952 as the thermal decomposition product of the azide N3F. It has the structure F−N=N−F and exists in both a cis- and trans-form.

Selenium monochloride is an inorganic compound with the formula Se2Cl2. Although it is called selenium monochloride, a more descriptive name might be diselenium dichloride. It is a reddish-brown, oily liquid that hydrolyses slowly. It exists in chemical equilibrium with SeCl2, SeCl4, chlorine, and elemental selenium. Selenium monochloride is mainly used as a reagent for the synthesis of Se-containing compounds.

In chemistry, chloryl refers to a triatomic cation with chemical formula ClO+
2. This species has the same general structure as chlorite (ClO−
2) but it is electronically different, with chlorine having a +5 oxidation state (rather than the +3 of chlorite). This makes it a rare example of a positively charged oxychloride. Chloryl compounds, such as FClO
2 and [ClO2][RuF6], are all highly reactive and react violently with water and most organic compounds.

Thionyl tetrafluoride is an inorganic compound gas with the formula SOF4. It is also known as sulfur tetrafluoride oxide. The shape of the molecule is a distorted trigonal bipyramid, with the oxygen found on the equator. The atoms on the equator have shorter bond lengths than the fluorine atoms on the axis. The sulfur oxygen bond is 1.409Å. A S−F bond on the axis has length 1.596Å and the S−F bond on the equator has length 1.539Å. The angle between the equatorial fluorine atoms is 112.8°. The angle between axial fluorine and oxygen is 97.7°. The angle between oxygen and equatorial fluorine is 123.6° and between axial and equatorial fluorine is 85.7°. The fluorine atoms only produce one NMR line, probably because they exchange positions.
Thiophosphoryl fluoride is an inorganic molecular gas with formula PSF3 containing phosphorus, sulfur and fluorine. It spontaneously ignites in air and burns with a cool flame. The discoverers were able to have flames around their hands without discomfort, and called it "probably one of the coldest flames known". The gas was discovered in 1888.
Chromium(II) fluoride is an inorganic compound with the formula CrF2. It exists as a blue-green iridescent solid. Chromium(II) fluoride is sparingly soluble in water, almost insoluble in alcohol, and is soluble in boiling hydrochloric acid, but is not attacked by hot distilled sulfuric acid or nitric acid. Like other chromous compounds, chromium(II) fluoride is oxidized to chromium(III) oxide in air.
Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar covalent bonds or ionic bonds. Most frequently, covalent bonds involving fluorine atoms are single bonds, although at least two examples of a higher order bond exist. Fluoride may act as a bridging ligand between two metals in some complex molecules. Molecules containing fluorine may also exhibit hydrogen bonding. Fluorine's chemistry includes inorganic compounds formed with hydrogen, metals, nonmetals, and even noble gases; as well as a diverse set of organic compounds. For many elements the highest known oxidation state can be achieved in a fluoride. For some elements this is achieved exclusively in a fluoride, for others exclusively in an oxide; and for still others the highest oxidation states of oxides and fluorides are always equal.
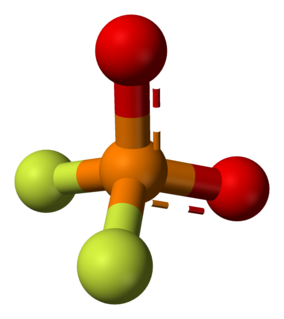
Difluorophosphate or difluorodioxophosphate or phosphorodifluoridate is an anion with formula PO
2F−
2. It has a single negative charge and resembles perchlorate (ClO−
4) and monofluorosulfonate (SO3F−) in shape and compounds. These ions are isoelectronic, along with tetrafluoroaluminate, phosphate, orthosilicate, and sulfate. It forms a series of compounds. The ion is toxic to mammals as it causes blockage to iodine uptake in the thyroid. However it is degraded in the body over several hours.
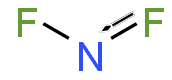
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer dinitrogen tetrafluoride.

Chlorine oxide trifluoride or chlorine trifluoride oxide is a corrosive liquid molecular compound with formula ClOF3. It was developed secretly as a rocket fuel oxidiser.