
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on haloimines and nitrones. Cyclic oximes and haloimines yield lactams.

Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Chloroacetophenone is thermally stable, and is the only tear agent that is distillable at ambient conditions.

In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and R' may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure R1C(=NOH)NR2R3.
Obidoxime is a member of the oxime family used to treat organophosphate poisoning. Oximes are drugs known for their ability to reverse the binding of organophosphorus compounds to the enzyme acetylcholinesterase (AChE).
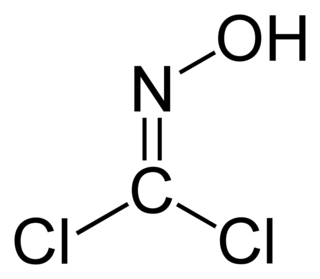
Phosgene oxime, or CX, is an organic compound with the formula Cl2C=N−OH. It is a potent chemical weapon, specifically a nettle agent. The compound itself is a colorless solid, but impure samples are often yellowish liquids. It has a strong, disagreeable and irritating odor. It is used as a reagent in organic chemistry.

Norelgestromin, or norelgestromine, sold under the brand names Evra and Ortho Evra among others, is a progestin medication which is used as a method of birth control for women. The medication is available in combination with an estrogen and is not available alone. It is used as a patch that is applied to the skin.
Nettle agents or urticants are a variety of chemical warfare agents that produce corrosive skin and tissue injury upon contact, resulting in erythema, urticaria, intense itching, and a hive-like rash.
Bromobenzyl cyanide (BBC), also known in the military idiom as camite (CA), is an obsolete lachrymatory agent introduced in World War I by the Allied Powers, being a standard agent, along with chloroacetophenone, adopted by the CWS. When implemented in World War I, it revolutionized the use of tear agents due to their extreme potency. BBC is toxic like chlorine gas.
In enzymology, an oximinotransferase is an enzyme that catalyzes the chemical reaction

Tulobuterol (INN) is a long-acting beta2-adrenergic receptor agonist, marketed in Japan as a transdermal patch under the name Hokunalin tape (ホクナリンテープ).

Milbemycin oxime, sold under the brand name Interceptor among others, is a veterinary medication from the group of milbemycins, used as a broad spectrum antiparasitic. It is active against worms (anthelmintic) and mites (miticide).

Asoxime chloride, or more commonly HI-6, is a Hagedorn oxime used in the treatment of organophosphate poisoning.
Trimedoxime bromide (INN), also known as dipyroxime or TMB-4, is an oxime used in the treatment of organophosphate poisoning It is chemically related to asoxime, pralidoxime, and obidoxime.

Phenaglycodol is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties. It is related pharmacologically to meprobamate, though it is not a carbamate.

Nisterime (INNTooltip International Nonproprietary Name), also known as 2α-chloro-4,5α-dihydrotestosterone 3-O-(p-nitrophenyl)oxime or as 2α-chloro-5α-androstan-17β-ol-3-one 3-O-(p-nitrophenyl)oxime, is a synthetic anabolic-androgenic steroid (AAS) and a derivative of dihydrotestosterone (DHT) that was never marketed. The C17α acetate ester of nisterime, nisterime acetate (ORF-9326), also exists and was developed as a postcoital contraceptive but was similarly never marketed.

P1-185, also known as progesterone 3-O-(L-valine)-E-oxime or as pregn-4-ene-3,20-dione 3-O-(L-valine)-E-oxime, is a synthetic progestogen and neurosteroid and an oxime ester analogue and prodrug of progesterone. It was developed as an improved water-soluble version of progesterone such that it could be formulated as an aqueous preparation and easily and rapidly administered intravenously as a potential therapy for traumatic brain injury. However, the chemical synthesis of P1-185 was described as somewhat challenging, so oxime conjugates of progesterone of the C20 instead of C3 position, such as EIDD-1723 and EIDD-036, have since been developed.
The Griesbaum coozonolysis is a name reaction in organic chemistry that allows for the preparation of tetrasubstituted ozonides (1,2,4-trioxolanes) by the reaction of O-methyl oximes with a carbonyl compound in the presence of ozone. Contrary to their usual roles as intermediates in ozonolysis and other oxidative alkene cleavage reactions, 1,2,4-trioxolanes are relatively stable compounds and are isolable.
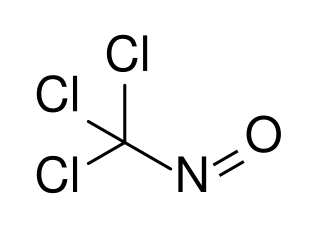
Trichloronitrosomethane is a chlorinated nitrosoalkane. It is a deep blue liquid with powerful lachrymatory effects.

Cholinesterase reactivators are drugs that reverse the inhibition of cholinesterase by organophosphates or sulfonates. They are used as antidote for treating organophosphate insecticide and nerve agent poisoning. Organophosphates are used industrially in agricultural pesticides, and globally as agents of chemical warfare. Discovered in 1955, Pralidoxime was the first cholinesterase reactivator used as an antidote to OP neurotoxicity and remains the most commonly used reactivator. Cholinesterase reactivators are indicated for anticholinesterase toxicity and cholinergic crisis, the signs of which are contained within the mnemonic DUMBELS : Diarrhea/diaphoresis, urinary frequency, miosis, bronchospasm/bronchorrhea, emesis, lacrimation, and salivation. Death may occur due to the blockage of nicotinic receptors of muscle fibers at the neuromuscular junction, causing paralysis of the muscles of respiration.













