| |||
| Names | |||
|---|---|---|---|
| IUPAC name Nitromethane | |||
| Preferred IUPAC name Nitromethane [1] | |||
| Other names Nitrocarbol | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.000.797 | ||
| KEGG | |||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
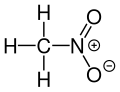
| CH3NO2 | |||
| Molar mass | 61.04 g/mol | ||
| Appearance | colorless, oily liquid [2] | ||
| Odor | Light, fruity [2] | ||
| Density | 1.1371 g/cm3 (20 °C) [3] | ||
| Melting point | −28.7 °C (−19.7 °F; 244.5 K) [3] | ||
| Boiling point | 101.2 °C (214.2 °F; 374.3 K) [3] | ||
| Critical point (T, P) | 588 K, 6.0 MPa [4] | ||
| ca. 10 g/100 mL | |||
| Solubility | miscible in diethyl ether, acetone, ethanol, methanol [3] | ||
| Vapor pressure | 28 mmHg (20 °C) [2] | ||
| Acidity (pKa) | |||
| −21.0·10−6 cm3/mol [7] | |||
| Thermal conductivity | 0.204 W/(m·K) at 25 °C [8] | ||
Refractive index (nD) | 1.3817 (20 °C) [3] | ||
| Viscosity | 0.63 cP at 25 °C [8] | ||
| 3.46 [9] | |||
| Explosive data | |||
| Shock sensitivity | Low | ||
| Friction sensitivity | Low | ||
| Detonation velocity | 6400 m/s | ||
| Thermochemistry [10] | |||
Heat capacity (C) | 106.6 J/(mol·K) | ||
Std molar entropy (S⦵298) | 171.8 J/(mol·K) | ||
Std enthalpy of formation (ΔfH⦵298) | −112.6 kJ/mol | ||
Gibbs free energy (ΔfG⦵) | −14.4 kJ/mol | ||
Std enthalpy of combustion (ΔcH⦵298) | -709 kJ/mol | ||
Enthalpy of fusion (ΔfH⦵fus) | 9.7 kJ/mol | ||
Enthalpy of vaporization (ΔfHvap) | 38.3 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards | Flammable, health hazard | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H203, H226, H301, H331, H351 | |||
| P210, P261, P280, P304+P340, P312, P370+P378, P403+P233 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 35 [9] °C (95 °F; 308 K) | ||
| 418 [9] °C (784 °F; 691 K) | |||
| Explosive limits | 7–22% [9] | ||
Threshold limit value (TLV) | 20 ppm [9] | ||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) | 940 mg/kg (oral, rat) 950 mg/kg (oral, mouse) [11] | ||
LDLo (lowest published) | 750 mg/kg (rabbit, oral) 125 mg/kg (dog, oral) [11] | ||
LCLo (lowest published) | 7087 ppm (mouse, 2 h) 1000 ppm (monkey) 2500 ppm (rabbit, 12 h) 5000 ppm (rabbit, 6 h) [11] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) | TWA 100 ppm (250 mg/m3) [2] | ||
REL (Recommended) | none [2] | ||
IDLH (Immediate danger) | 750 ppm [2] | ||
| Related compounds | |||
Related nitro compounds | nitroethane | ||
Related compounds | methyl nitrite methyl nitrate | ||
| Supplementary data page | |||
| Nitromethane (data page) | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Nitromethane, sometimes shortened to simply "nitro", is an organic compound with the chemical formula CH3NO2. It is the simplest organic nitro compound. It is a polar liquid commonly used as a solvent in a variety of industrial applications such as in extractions, as a reaction medium, and as a cleaning solvent. As an intermediate in organic synthesis, it is used widely in the manufacture of pesticides, explosives, fibers, and coatings. [12] Nitromethane is used as a fuel additive in various motorsports and hobbies, e.g. Top Fuel drag racing and miniature internal combustion engines in radio control, control line and free flight model aircraft.