
Mass spectrometry (MS) is an analytical technique that is used to measure the mass-to-charge ratio of ions. The results are presented as a mass spectrum, a plot of intensity as a function of the mass-to-charge ratio. Mass spectrometry is used in many different fields and is applied to pure samples as well as complex mixtures.

An ion source is a device that creates atomic and molecular ions. Ion sources are used to form ions for mass spectrometers, optical emission spectrometers, particle accelerators, ion implanters and ion engines.

Electron ionization is an ionization method in which energetic electrons interact with solid or gas phase atoms or molecules to produce ions. EI was one of the first ionization techniques developed for mass spectrometry. However, this method is still a popular ionization technique. This technique is considered a hard ionization method, since it uses highly energetic electrons to produce ions. This leads to extensive fragmentation, which can be helpful for structure determination of unknown compounds. EI is the most useful for organic compounds which have a molecular weight below 600. Also, several other thermally stable and volatile compounds in solid, liquid and gas states can be detected with the use of this technique when coupled with various separation methods.

Melamine is an organic compound with the formula C3H6N6. This white solid is a trimer of cyanamide, with a 1,3,5-triazine skeleton. Like cyanamide, it contains 66% nitrogen by mass, and its derivatives have fire-retardant properties due to its release of nitrogen gas when burned or charred. Melamine can be combined with formaldehyde and other agents to produce melamine resins. Such resins are characteristically durable thermosetting plastic used in high pressure decorative laminates such as Formica, melamine dinnerware including cooking utensils, plates, plastic products, laminate flooring, and dry erase boards. Melamine foam is used as insulation, soundproofing material and in polymeric cleaning products, such as Magic Eraser.

Metabolomics is the scientific study of chemical processes involving metabolites, the small molecule substrates, intermediates, and products of cell metabolism. Specifically, metabolomics is the "systematic study of the unique chemical fingerprints that specific cellular processes leave behind", the study of their small-molecule metabolite profiles. The metabolome represents the complete set of metabolites in a biological cell, tissue, organ, or organism, which are the end products of cellular processes. Messenger RNA (mRNA), gene expression data, and proteomic analyses reveal the set of gene products being produced in the cell, data that represents one aspect of cellular function. Conversely, metabolic profiling can give an instantaneous snapshot of the physiology of that cell, and thus, metabolomics provides a direct "functional readout of the physiological state" of an organism. There are indeed quantifiable correlations between the metabolome and the other cellular ensembles, which can be used to predict metabolite abundances in biological samples from, for example mRNA abundances. One of the ultimate challenges of systems biology is to integrate metabolomics with all other -omics information to provide a better understanding of cellular biology.

In mass spectrometry, matrix-assisted laser desorption/ionization (MALDI) is an ionization technique that uses a laser energy-absorbing matrix to create ions from large molecules with minimal fragmentation. It has been applied to the analysis of biomolecules and various organic molecules, which tend to be fragile and fragment when ionized by more conventional ionization methods. It is similar in character to electrospray ionization (ESI) in that both techniques are relatively soft ways of obtaining ions of large molecules in the gas phase, though MALDI typically produces far fewer multi-charged ions.

Atmospheric pressure chemical ionization (APCI) is an ionization method used in mass spectrometry which utilizes gas-phase ion-molecule reactions at atmospheric pressure (105 Pa), commonly coupled with high-performance liquid chromatography (HPLC). APCI is a soft ionization method similar to chemical ionization where primary ions are produced on a solvent spray. The main usage of APCI is for polar and relatively less polar thermally stable compounds with molecular weight less than 1500 Da. The application of APCI with HPLC has gained a large popularity in trace analysis detection such as steroids, pesticides and also in pharmacology for drug metabolites.
In mass spectrometry, direct analysis in real time (DART) is an ion source that produces electronically or vibronically excited-state species from gases such as helium, argon, or nitrogen that ionize atmospheric molecules or dopant molecules. The ions generated from atmospheric or dopant molecules undergo ion-molecule reactions with the sample molecules to produce analyte ions. Analytes with low ionization energy may be ionized directly. The DART ionization process can produce positive or negative ions depending on the potential applied to the exit electrode.

Desorption electrospray ionization (DESI) is an ambient ionization technique that can be coupled to mass spectrometry (MS) for chemical analysis of samples at atmospheric conditions. Coupled ionization sources-MS systems are popular in chemical analysis because the individual capabilities of various sources combined with different MS systems allow for chemical determinations of samples. DESI employs a fast-moving charged solvent stream, at an angle relative to the sample surface, to extract analytes from the surfaces and propel the secondary ions toward the mass analyzer. This tandem technique can be used to analyze forensics analyses, pharmaceuticals, plant tissues, fruits, intact biological tissues, enzyme-substrate complexes, metabolites and polymers. Therefore, DESI-MS may be applied in a wide variety of sectors including food and drug administration, pharmaceuticals, environmental monitoring, and biotechnology.
Robert Graham Cooks is the Henry Bohn Hass Distinguished Professor of Chemistry in the Aston Laboratories for Mass Spectrometry at Purdue University. He is an ISI Highly Cited Chemist, with over 1,000 publications and an H-index of 144.
Michael L. Gross is Professor of Chemistry, Medicine, and Immunology, at Washington University in St. Louis. He was formerly Professor of Chemistry at the University of Nebraska-Lincoln from 1968–1994. He is recognized for his contributions to the field of mass spectrometry and ion chemistry. He is credited with the discovery of distonic ions, chemical species containing a radical and an ionic site on different atoms of the same molecule.

Ion mobility spectrometry–mass spectrometry (IMS-MS) is an analytical chemistry method that separates gas phase ions based on their interaction with a collision gas and their masses. In the first step, the ions are separated according to their mobility through a buffer gas on a millisecond timescale using an ion mobility spectrometer. The separated ions are then introduced into a mass analyzer in a second step where their mass-to-charge ratios can be determined on a microsecond timescale. The effective separation of analytes achieved with this method makes it widely applicable in the analysis of complex samples such as in proteomics and metabolomics.

Methyldichloroarsine, sometimes abbreviated "MD" and also known as methyl Dick, is an organoarsenic compound with the formula CH3AsCl2. This colourless volatile liquid is a highly toxic vesicant that has been used in chemical warfare.

Ambient ionization is a form of ionization in which ions are formed in an ion source outside the mass spectrometer without sample preparation or separation. Ions can be formed by extraction into charged electrospray droplets, thermally desorbed and ionized by chemical ionization, or laser desorbed or ablated and post-ionized before they enter the mass spectrometer.

Instrumental analysis is a field of analytical chemistry that investigates analytes using scientific instruments.

Laser ablation electrospray ionization (LAESI) is an ambient ionization method for mass spectrometry that combines laser ablation from a mid-infrared (mid-IR) laser with a secondary electrospray ionization (ESI) process. The mid-IR laser is used to generate gas phase particles which are then ionized through interactions with charged droplets from the ESI source. LAESI was developed in Professor Akos Vertes lab by Peter Nemes in 2007 and it was marketed commercially by Protea Biosciences, Inc until 2017. Fiber-LAESI for single-cell analysis approach was developed by Bindesh Shrestha in Professor Vertes lab in 2009. LAESI is a novel ionization source for mass spectrometry (MS) that has been used to perform MS imaging of plants, tissues, cell pellets, and even single cells. In addition, LAESI has been used to analyze historic documents and untreated biofluids such as urine and blood. The technique of LAESI is performed at atmospheric pressure and therefore overcomes many of the obstacles of traditional MS techniques, including extensive and invasive sample preparation steps and the use of high vacuum. Because molecules and aerosols are ionized by interacting with an electrospray plume, LAESI's ionization mechanism is similar to SESI and EESI techniques.
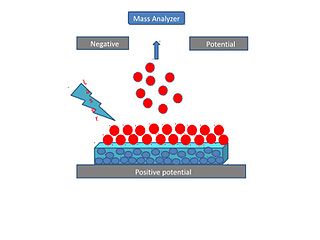
Surface-assisted laser desorption/ionization (SALDI) is a soft laser desorption technique used for mass spectrometry analysis of biomolecules, polymers, and small organic molecules. In its first embodiment Koichi Tanaka used a cobalt/glycerol liquid matrix and subsequent applications included a graphite/glycerol liquid matrix as well as a solid surface of porous silicon. The porous silicon represents the first matrix-free SALDI surface analysis allowing for facile detection of intact molecular ions, these porous silicon surfaces also facilitated the analysis of small molecules at the yoctomole level. At present laser desorption/ionization methods using other inorganic matrices such as nanomaterials are often regarded as SALDI variants. As an example, silicon nanowires as well as Titania nanotube arrays (NTA) have been used as substrates to detect small molecules. SALDI is used to detect proteins and protein-protein complexes. A related method named "ambient SALDI" - which is a combination of conventional SALDI with ambient mass spectrometry incorporating the direct analysis real time (DART) ion source has also been demonstrated. SALDI is considered one of the most important techniques in MS and has many applications.
Time-resolved mass spectrometry (TRMS) is a strategy in analytical chemistry that uses mass spectrometry platform to collect data with temporal resolution. Implementation of TRMS builds on the ability of mass spectrometers to process ions within sub-second duty cycles. It often requires the use of customized experimental setups. However, they can normally incorporate commercial mass spectrometers. As a concept in analytical chemistry, TRMS encompasses instrumental developments, methodological developments, and applications.

Secondary electro-spray ionization (SESI) is an ambient ionization technique for the analysis of trace concentrations of vapors, where a nano-electrospray produces charging agents that collide with the analyte molecules directly in gas-phase. In the subsequent reaction, the charge is transferred and vapors get ionized, most molecules get protonated and deprotonated. SESI works in combination with mass spectrometry or ion-mobility spectrometry.
















