Nerve agents, sometimes also called nerve gases, are a class of organic chemicals that disrupt the mechanisms by which nerves transfer messages to organs. The disruption is caused by the blocking of acetylcholinesterase (AChE), an enzyme that catalyzes the breakdown of acetylcholine, a neurotransmitter. Nerve agents are irreversible acetylcholinesterase inhibitors used as poison.

Sarin is an extremely toxic organophosphorus compound. A colourless, odourless liquid, it is used as a chemical weapon due to its extreme potency as a nerve agent. Exposure can be lethal even at very low concentrations, where death can occur within one to ten minutes after direct inhalation of a lethal dose, due to suffocation from respiratory paralysis, unless antidotes are quickly administered. People who absorb a non-lethal dose and do not receive immediate medical treatment may suffer permanent neurological damage.

Tabun is an extremely toxic compound of the organophosphate family. It is not present in nature. At room temperature, the pure compound is a clear and viscous liquid. However, impurities imparted during its manufacture are almost always present, turning it into a yellow or brown liquid. Exposed to environs, it slowly volatizes into the atmosphere, with the vapor having a slight fruity or almond-like odor. As the compound has a much higher molecular mass compared to air, Tabun gas tends to accumulate in low-lying areas.

VX is an extremely toxic synthetic chemical compound in the organophosphorus class, specifically, a thiophosphonate. In the class of nerve agents, it was developed for military use in chemical warfare after translation of earlier discoveries of organophosphate toxicity in pesticide research. In its pure form, VX is an oily, relatively non-volatile liquid that is amber-like in colour. Because of its low volatility, VX persists in environments where it is dispersed.

The enzyme cholinesterase (EC 3.1.1.8, choline esterase; systematic name acylcholine acylhydrolase) catalyses the hydrolysis of choline-based esters:
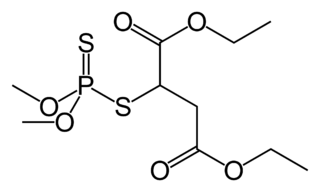
Malathion is an organophosphate insecticide which acts as an acetylcholinesterase inhibitor. In the USSR, it was known as carbophos, in New Zealand and Australia as maldison and in South Africa as mercaptothion.
Cyclosarin or GF is an extremely toxic substance used as a chemical weapon. It is a member of the G-series family of nerve agents, a group of chemical weapons discovered and synthesized by a German team led by Gerhard Schrader. The major nerve gases are the G agents, sarin (GB), soman (GD), tabun (GA), and the V agents such as VX. The original agent, tabun, was discovered in Germany in 1936 in the process of work on organophosphorus insecticides. Next came sarin, soman and finally, cyclosarin, a product of commercial insecticide laboratories prior to World War II.

Ethion (C9H22O4P2S4) is an organophosphate insecticide. It is known to affect the neural enzyme acetylcholinesterase and disrupt its function.

Huperzine A is a naturally-occurring sesquiterpene alkaloid compound found in the firmoss Huperzia serrata and in varying quantities in other food Huperzia species, including H. elmeri, H. carinat, and H. aqualupian. Huperzine A has been investigated as a treatment for neurological conditions such as Alzheimer's disease, but a 2013 meta-analysis of those studies concluded that they were of poor methodological quality and the findings should be interpreted with caution. Huperzine A inhibits the breakdown of the neurotransmitter acetylcholine (ACh) by the enzyme acetylcholinesterase. It is also an antagonist of the NMDA-receptor. It is commonly available over the counter as a nutritional supplement and marketed as a memory and concentration enhancer.
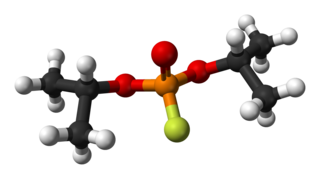
Diisopropyl fluorophosphate (DFP) or Isoflurophate is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine and as an organophosphorus insecticide. It is stable, but undergoes hydrolysis when subjected to moisture.

Azinphos-methyl (Guthion) is a broad spectrum organophosphate insecticide manufactured by Bayer CropScience, Gowan Co., and Makhteshim Agan. Like other pesticides in this class, it owes its insecticidal properties to the fact that it is an acetylcholinesterase inhibitor. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act, and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities.
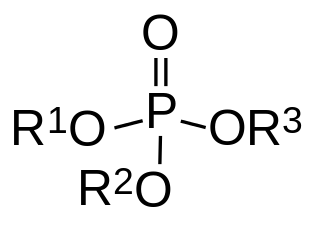
Organophosphate poisoning is poisoning due to organophosphates (OPs). Organophosphates are used as insecticides, medications, and nerve agents. Symptoms include increased saliva and tear production, diarrhea, vomiting, small pupils, sweating, muscle tremors, and confusion. While onset of symptoms is often within minutes to hours, some symptoms can take weeks to appear. Symptoms can last for days to weeks.

Acetylcholinesterase (HGNC symbol ACHE; EC 3.1.1.7; systematic name acetylcholine acetylhydrolase), also known as AChE, AChase or acetylhydrolase, is the primary cholinesterase in the body. It is an enzyme that catalyzes the breakdown of acetylcholine and some other choline esters that function as neurotransmitters:
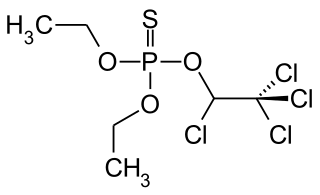
Chlorethoxyfos is an organophosphate acetylcholinesterase inhibitor used as an insecticide. It is registered for the control of corn rootworms, wireworms, cutworms, seed corn maggot, white grubs and symphylans on corn. The insecticide is sold under the trade name Fortress by E.I. du Pont de Nemours & Company.

Acetylcholinesterase inhibitors (AChEIs) also often called cholinesterase inhibitors, inhibit the enzyme acetylcholinesterase from breaking down the neurotransmitter acetylcholine into choline and acetate, thereby increasing both the level and duration of action of acetylcholine in the central nervous system, autonomic ganglia and neuromuscular junctions, which are rich in acetylcholine receptors. Acetylcholinesterase inhibitors are one of two types of cholinesterase inhibitors; the other being butyryl-cholinesterase inhibitors. Acetylcholinesterase is the primary member of the cholinesterase enzyme family.

Ethoprophos (or ethoprop) is an organophosphate ester with the formula C8H19O2PS2. It is a clear yellow to colourless liquid that has a characteristic mercaptan-like odour. It is used as an insecticide and nematicide and it is an acetylcholinesterase inhibitor.
Methanesulfonyl fluoride (MSF) has long been known to be a potent inhibitor of acetylcholinesterase (AChE), the enzyme that regulates acetylcholine, an important neurotransmitter in both the central and peripheral nervous systems.
IDFP is an organophosphorus compound related to the nerve agent sarin.

Cholinesterase reactivators are drugs that reverse the inhibition of cholinesterase by organophosphates or sulfonates. They are used as antidote for treating organophosphate insecticide and nerve agent poisoning. Organophosphates are used industrially in agricultural pesticides, and globally as agents of chemical warfare. Discovered in 1955, Pralidoxime was the first cholinesterase reactivator used as an antidote to OP neurotoxicity and remains the most commonly used reactivator. Cholinesterase reactivators are indicated for anticholinesterase toxicity and cholinergic crisis, the signs of which are contained within the mnemonic DUMBELS : Diarrhea/diaphoresis, urinary frequency, miosis, bronchospasm/bronchorrhea, emesis, lacrimation, and salivation. Death may occur due to the blockage of nicotinic receptors of muscle fibers at the neuromuscular junction, causing paralysis of the muscles of respiration.