A cyclic nucleotide (cNMP) is a single-phosphate nucleotide with a cyclic bond arrangement between the sugar and phosphate groups. Like other nucleotides, cyclic nucleotides are composed of three functional groups: a sugar, a nitrogenous base, and a single phosphate group. As can be seen in the cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) images, the 'cyclic' portion consists of two bonds between the phosphate group and the 3' and 5' hydroxyl groups of the sugar, very often a ribose.
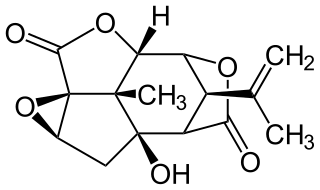
Picrotoxin, also known as cocculin, is a poisonous crystalline plant compound. It was first isolated by the French pharmacist and chemist Pierre François Guillaume Boullay (1777–1869) in 1812. The name "picrotoxin" is a combination of the Greek words "picros" (bitter) and "toxicon" (poison). A mixture of two different compounds, picrotoxin occurs naturally in the fruit of the Anamirta cocculus plant, although it can also be synthesized chemically.

Cyclic nucleotide–gated ion channels or CNG channels are ion channels that function in response to the binding of cyclic nucleotides. CNG channels are nonselective cation channels that are found in the membranes of various tissue and cell types, and are significant in sensory transduction as well as cellular development. Their function can be the result of a combination of the binding of cyclic nucleotides and either a depolarization or a hyperpolarization event. Initially discovered in the cells that make up the retina of the eye, CNG channels have been found in many different cell types across both the animal and the plant kingdoms. CNG channels have a very complex structure with various subunits and domains that play a critical role in their function. CNG channels are significant in the function of various sensory pathways including vision and olfaction, as well as in other key cellular functions such as hormone release and chemotaxis. CNG channels have also been found to exist in prokaryotes, including many spirochaeta, though their precise role in bacterial physiology remains unknown.

The GABAA receptor (GABAAR) is an ionotropic receptor and ligand-gated ion channel. Its endogenous ligand is γ-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the central nervous system. Accurate regulation of GABAergic transmission through appropriate developmental processes, specificity to neural cell types, and responsiveness to activity is crucial for the proper functioning of nearly all aspects of the central nervous system (CNS). Upon opening, the GABAA receptor on the postsynaptic cell is selectively permeable to chloride ions and, to a lesser extent, bicarbonate ions.

Tetramethylenedisulfotetramine (TETS) is an organic compound used as a rodenticide. It is an odorless, tasteless white powder that is slightly soluble in water, DMSO and acetone, and insoluble in methanol and ethanol. It is a sulfamide derivative. It can be synthesized by reacting sulfamide with formaldehyde solution in acidified water. When crystallized from acetone, it forms cubic crystals with a melting point of 255–260 °C.

A GABA receptor agonist is a drug that is an agonist for one or more of the GABA receptors, producing typically sedative effects, and may also cause other effects such as anxiolytic, anticonvulsant, and muscle relaxant effects. There are three receptors of the gamma-aminobutyric acid. The two receptors GABA-α and GABA-ρ are ion channels that are permeable to chloride ions which reduces neuronal excitability. The GABA-β receptor belongs to the class of G-Protein coupled receptors that inhibit adenylyl cyclase, therefore leading to decreased cyclic adenosine monophosphate (cAMP). GABA-α and GABA-ρ receptors produce sedative and hypnotic effects and have anti-convulsion properties. GABA-β receptors also produce sedative effects. Furthermore, they lead to changes in gene transcription.
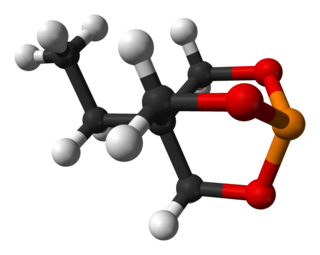
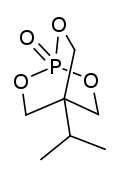
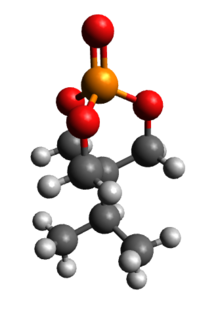
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a phosphite ester used as a ligand in organometallic chemistry. Trimethylolpropane phosphite is sometimes abbreviated to EtCage. It is a white solid that is soluble in organic solvents. It is also highly toxic.
Hyperpolarization-activated cyclic nucleotide–gated (HCN) channels are integral membrane proteins that serve as nonselective voltage-gated cation channels in the plasma membranes of heart and brain cells. HCN channels are sometimes referred to as pacemaker channels because they help to generate rhythmic activity within groups of heart and brain cells. HCN channels are activated by membrane hyperpolarization, are permeable to Na + and K +, and are constitutively open at voltages near the resting membrane potential. HCN channels are encoded by four genes and are widely expressed throughout the heart and the central nervous system.
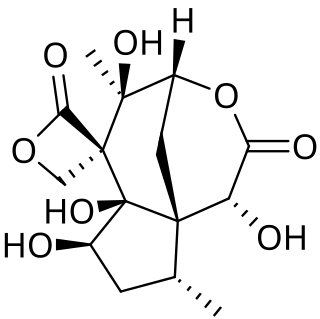
Anisatin is an extremely toxic, insecticidally active component of the shikimi plant. The lethal dose is 1 mg/kg (i.p.) in mice. Symptoms begin to appear about 1–6 hours after ingestion, beginning with gastrointestinal ailments, such as diarrhea, vomiting, and stomach pain, followed by nervous system excitation, seizures, loss of consciousness, and respiratory paralysis, which is the ultimate cause of death.

Etazolate (SQ-20,009, EHT-0202) is an anxiolytic drug which is a pyrazolopyridine derivative and has unique pharmacological properties. It acts as a positive allosteric modulator of the GABAA receptor at the barbiturate binding site, as an adenosine antagonist of the A1 and A2 subtypes, and as a phosphodiesterase inhibitor selective for the PDE4 isoform. It is currently in clinical trials for the treatment of Alzheimer's disease.

Flurothyl (Indoklon) is a volatile liquid drug from the halogenated ether family, related to inhaled anaesthetic agents such as diethyl ether, but having the opposite effects, acting as a stimulant and convulsant. A clear and stable liquid, it has a mild ethereal odor whose vapors are non-flammable. It is excreted from the body by the lungs in an unchanged state.
A convulsant is a drug which induces convulsions and/or epileptic seizures, the opposite of an anticonvulsant. These drugs generally act as stimulants at low doses, but are not used for this purpose due to the risk of convulsions and consequent excitotoxicity. Most convulsants are antagonists at either the GABAA or glycine receptors, or ionotropic glutamate receptor agonists. Many other drugs may cause convulsions as a side effect at high doses but only drugs whose primary action is to cause convulsions are known as convulsants. Nerve agents such as sarin, which were developed as chemical weapons, produce convulsions as a major part of their toxidrome, but also produce a number of other effects in the body and are usually classified separately. Dieldrin which was developed as an insecticide blocks chloride influx into the neurons causing hyperexcitability of the CNS and convulsions. The Irwin observation test and other studies that record clinical signs are used to test the potential for a drug to induce convulsions. Camphor, and other terpenes given to children with colds can act as convulsants in children who have had febrile seizures.

Tutin is a poisonous plant derivative found in New Zealand tutu plants. It acts as a potent antagonist of the glycine receptor, and has powerful convulsant effects. It is used in scientific research into the glycine receptor. It is sometimes associated with outbreaks of toxic honey poisoning when bees feed on honeydew exudate from the sap-sucking passion vine hopper insect, when the vine hoppers have been feeding on the sap of tutu bushes. Toxic honey is a rare event and is more likely to occur when comb honey is eaten directly from a hive that has been harvesting honeydew from passionvine hoppers feeding on tutu plants.

Pentylenetetrazol, also known as pentylenetetrazole, leptazol, metrazol, pentetrazol (INN), pentamethylenetetrazol, Corazol, Cardiazol, Deumacard, or PTZ, is a drug formerly used as a circulatory and respiratory stimulant. High doses cause convulsions, as discovered by Hungarian-American neurologist and psychiatrist Ladislas J. Meduna in 1934. It has been used in convulsive therapy, and was found to be effective—primarily for depression—but side effects such as uncontrolled seizures were difficult to avoid. In 1939, pentylenetetrazol was replaced by electroconvulsive therapy, which is easier to administer, as the preferred method for inducing seizures in England's mental hospitals. In the US, its approval by the Food and Drug Administration was revoked in 1982. It is used in Italy as a cardio-respiratory stimulant in combination with codeine in a cough suppressant drug.

Dioscorine is an alkaloid toxin isolated from the tubers of tropical yam on several continents. It has been used as a monkey poison in some African countries, and as an arrow poison to aid in hunting in several parts of Asia. It was first isolated from Dioscorea hirsute by Boorsma in 1894 and obtained in a crystalline form by Schutte in 1897, and has since been found in other Dioscorea species. Dioscorine is a neurotoxin that acts by blocking the nicotinic acetylcholine receptor. Dioscorine is generally isolated in tandem with other alkaloids such as dioscin but is usually the most potent toxin in the mixture. It is a convulsant, producing symptoms similar to picrotoxin, with which it shares a similar mechanism of action.
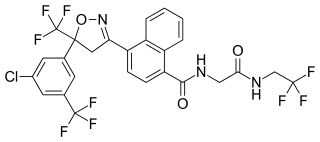
Afoxolaner (INN) is an insecticide and acaricide that belongs to the isoxazoline chemical compound group.

TBPS (tert-butylbicyclophosphorothionate) is a bicyclic phosphate convulsant. It is an extremely potent GABA receptor antagonist.
1-(4-Chlorophenyl)silatrane is an extremely toxic organosilicon compound which was developed by M&T Chemicals as a single-dose rodenticide. It was never registered as rodenticide, except for experimental use. 1-(4-Chlorophenyl)silatrane was one of the chemicals studied in the Project Coast.

TBPO is an extremely toxic bicyclic phosphate convulsant and GABA receptor antagonist. It is the most toxic bicyclic phosphate known, with an LD50 of 36 μg/kg in mice.
Bicyclic phosphate is a class of organophosphate compounds that are used as flame retardants, stabilizers and antioxidants. They are also used in spectroscopic studies.