
Charybdotoxin (ChTX) is a 37 amino acid neurotoxin from the venom of the scorpion Leiurus quinquestriatus hebraeus (deathstalker) that blocks calcium-activated potassium channels. This blockade causes hyperexcitability of the nervous system. It is a close homologue of agitoxin and both toxins come from Leiurus quinquestriatus hebraeus. It is named after Charybdis, a sea monster from Greek myth.

Defensins are small cysteine-rich cationic proteins across cellular life, including vertebrate and invertebrate animals, plants, and fungi. They are host defense peptides, with members displaying either direct antimicrobial activity, immune signaling activities, or both. They are variously active against bacteria, fungi and many enveloped and nonenveloped viruses. They are typically 18-45 amino acids in length, with three or four highly conserved disulphide bonds.

A conotoxin is one of a group of neurotoxic peptides isolated from the venom of the marine cone snail, genus Conus.

Antimicrobial peptides (AMPs), also called host defence peptides (HDPs) are part of the innate immune response found among all classes of life. Fundamental differences exist between prokaryotic and eukaryotic cells that may represent targets for antimicrobial peptides. These peptides are potent, broad spectrum antimicrobials which demonstrate potential as novel therapeutic agents. Antimicrobial peptides have been demonstrated to kill Gram negative and Gram positive bacteria, enveloped viruses, fungi and even transformed or cancerous cells. Unlike the majority of conventional antibiotics it appears that antimicrobial peptides frequently destabilize biological membranes, can form transmembrane channels, and may also have the ability to enhance immunity by functioning as immunomodulators.

The Chinese red-headed centipede, also known as the Chinese red head, is a centipede from East Asia. It averages 20 cm (8 in) in length and lives in damp environments.
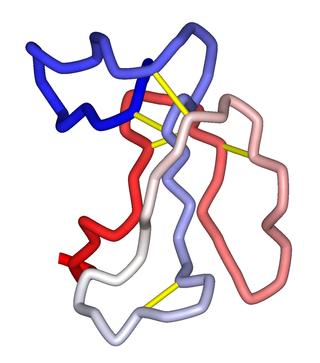
α-Bungarotoxin is one of the bungarotoxins, components of the venom of the elapid Taiwanese banded krait snake. It is a type of α-neurotoxin, a neurotoxic protein that is known to bind competitively and in a relatively irreversible manner to the nicotinic acetylcholine receptor found at the neuromuscular junction, causing paralysis, respiratory failure, and death in the victim. It has also been shown to play an antagonistic role in the binding of the α7 nicotinic acetylcholine receptor in the brain, and as such has numerous applications in neuroscience research.

Dermcidin is a protein with 110 amino acids that in humans is encoded by the DCD gene. The full-length protein produces derived peptides as proteolysis-inducing factor (PIF) and other anti-microbial peptides, secreted by human eccrine sweat glands onto the skin as a part of the innate host defense of the immune system. PIF is involved in muscular proteolysis.

Scolopendra subspinipes is a species of very large centipede found throughout southeastern Asia. One of the most widespread and common species in the genus Scolopendra, it is also found on virtually all land areas around and within the Indian Ocean, all of tropical and subtropical Asia from Russia to the islands of Malaysia and Indonesia, Australia, South and Central America, the Caribbean islands, and possibly parts of the southern United States, but how much of this range is natural and how much due to human introduction is unclear. With a wide geographic range and numerous color variations, the species is known by many common names, including jungle centipede, orange-legged centipede, Hawaiian centipede, and Vietnamese centipede.

Scolopendra polymorpha, the common desert centipede, tiger centipede, banded desert centipede, or Sonoran Desert centipede, is a centipede species found in western North America and the Hawaiian Islands.

Arthropod defensins are a family defensin proteins found in mollusks, insects, and arachnids. These cysteine-rich antibacterial peptides are primarily active against Gram-positive bacteria and fungi in vitro. However Drosophila fruit flies mutant for the fly defensin were more susceptible to infection by the Gram-negative bacteria Providencia burhodogranariea, and resisted infection against Gram-positive bacteria like wild-type flies. It remains to be seen how in vitro activity relates to in vivo function. Mutants for the defensin-like antimicrobial peptide Drosomycin were more susceptible to fungi, validating a role for defensin-like peptides in anti-fungal defence.
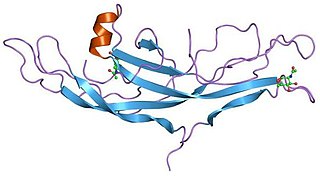
A cystine knot is a protein structural motif containing three disulfide bridges. The sections of polypeptide that occur between two of them form a loop through which a third disulfide bond passes, forming a rotaxane substructure. The cystine knot motif stabilizes protein structure and is conserved in proteins across various species. There are three types of cystine knot, which differ in the topology of the disulfide bonds:

Cecropins are antimicrobial peptides. They were first isolated from the hemolymph of Hyalophora cecropia, whence the term cecropin was derived. Cecropins lyse bacterial cell membranes; they also inhibit proline uptake and cause leaky membranes.

Scolopendra morsitans, also known as the Tanzanian blue ringleg or red-headed centipede, is a species of centipede in the family Scolopendridae. S. morsitans is the type species for the genus Scolopendra.

Guangxitoxin, also known as GxTX, is a peptide toxin found in the venom of the tarantula Plesiophrictus guangxiensis. It primarily inhibits outward voltage-gated Kv2.1 potassium channel currents, which are prominently expressed in pancreatic β-cells, thus increasing insulin secretion.
Atrolysin A is an enzyme that is one of six hemorrhagic toxins found in the venom of western diamondback rattlesnake. This endopeptidase has a length of 419 amino acid residues. The metalloproteinase disintegrin-like domain and the cysteine-rich domain of the enzyme are responsible for the enzyme's hemorrhagic effects on organisms via inhibition of platelet aggregation.
Ssm6a, or μ-SLPTX-Ssm6a, is a toxin from the venom of the Chinese red-headed centipede. It has strong analgesic properties, probably owing to its strong inhibitory effects on Nav1.7 channels.
RhTx is a small peptide toxin from Scolopendra subspinipes mutilans, also called the Chinese red-headed centipede. RhTx binds to the outer pore region of the temperature regulated TRPV1 ion channel, preferably in activated state, causing a downwards shift in the activation threshold temperature, which leads to the immediate onset of heat pain.

Double-knot toxin (DkTx), also known as Tau-theraphotoxin-Hs1a or Tau-TRTX-Hs1a, is a toxin found in the venom of the Chinese Bird spider, a tarantula species primarily living in the Guangxi province of China. This toxin, characterized by its bivalent structure of two Inhibitor Cysteine Knots (ICK), is thought to induce excruciating and long-lasting pain by activating the transient receptor potential vanilloid 1 (TRPV1) channel.

Grammostola mechanotoxin #4, also known as M-theraphotoxin-Gr1a (M-TRTX-Gr1a), is a neurotoxin isolated from the venom of the spider Chilean rose tarantula Grammostola spatulate. This amphiphilic peptide, which consists of 35 amino acids, belongs to the inhibitory cysteine knot (ICK) peptide family. It reduces mechanical sensation by inhibiting mechanosensitive channels (MSCs).
Phlotoxin is a neurotoxin from the venom of the tarantula Phlogiellus that targets mostly voltage-sensitive sodium channels and mainly Nav1.7. The only non-sodium voltage-sensitive channel that is inhibited by Phlotoxin is Kv3.4. Nav1.4 and Nav1.6 seem to be Phlotoxin-1-sensitive to some extent as well.
















