 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Motilium, others |
| Other names | R-33812; R33812; KW-5338; KW5338; NSC-299589; NSC299589 |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | By mouth, rectal [2] |
| Drug class | D2 receptor antagonist; Prolactin releaser |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: 13–17% [2] [6] Intramuscular: 90% [2] |
| Protein binding | ~92% [2] |
| Metabolism | Hepatic (CYP3A4/5) and intestinal (first-pass) [2] [7] |
| Metabolites | All inactive [2] [7] |
| Onset of action | 30–60 minutes [8] |
| Elimination half-life | 7–9 hours [9] [2] [6] |
| Excretion | Feces: 66% [2] Urine: 32% [2] Breast milk: small quantities [2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.055.408 |
| Chemical and physical data | |
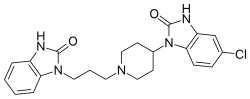
| Formula | C22H24ClN5O2 |
| Molar mass | 425.92 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 242.5 °C (468.5 °F) |
| |
| |
| (verify) | |
Domperidone, sold under the brand name Motilium among others, is a dopamine antagonist medication which is used to treat nausea and vomiting and certain gastrointestinal problems like gastroparesis (delayed gastric emptying). It raises the level of prolactin in the human body. [2] [10] It may be taken by mouth or rectally. [2] [11] [12]
Contents
- Medical uses
- Nausea and vomiting
- Gastroparesis
- Lactation
- Other uses
- Available forms
- Veterinary uses
- Contraindications
- Side effects
- Elevated prolactin levels
- Rare reactions
- Interactions
- Pharmacology
- Pharmacodynamics
- Pharmacokinetics
- Chemistry
- History
- Society and culture
- Generic names
- Regulatory approval
- Formulations
- Research
- References
Side effects may include headache, anxiety, dry mouth, abdominal cramps, diarrhea, and elevated prolactin levels. [13] [2] [10] [14] Secondary to increased prolactin levels, breast changes, milk outflow, menstrual irregularities, and hypogonadism can occur. [2] [10] [14] Domperidone may also cause QT prolongation and has rarely been associated with serious cardiac complications such as sudden cardiac death. [15] [16] [17] [18] However, the risks are small and occur more with high doses. [18] [19] Domperidone acts as a peripherally selective antagonist of the dopamine D2 and D3 receptors. [2] [10] Due to its low entry into the brain, the side effects of domperidone are different from those of other dopamine receptor antagonists like metoclopramide and it produces little in the way of central nervous system adverse effects. [2] [10] However, domperidone can nonetheless increase prolactin levels as the pituitary gland is outside of the blood–brain barrier. [20]
Domperidone was discovered in 1974 and was introduced for medical use in 1979. [21] [22] [23] It was developed by Janssen Pharmaceutica. [21] [22] Domperidone is available over-the-counter in many countries, for instance in Europe and elsewhere throughout the world. [24] [2] It is not approved for use in the United States. [25] [26] [2] However, it is available in the United States for people with severe and treatment-refractory gastrointestinal motility problems under an expanded access individual-patient investigational new drug application. [25] An analogue of domperidone called deudomperidone is under development for potential use in the United States and other countries. [27] [28] [29]