Related Research Articles

An analgesic or painkiller is any member of the group of drugs used to achieve analgesia, relief from pain. They are distinct from anesthetics, which temporarily affect, and in some instances eliminate, sensation.
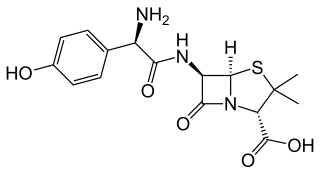
Amoxicillin is an antibiotic used to treat a number of bacterial infections. These include middle ear infection, strep throat, pneumonia, skin infections, and urinary tract infections among others. It is taken by mouth, or less commonly by injection.
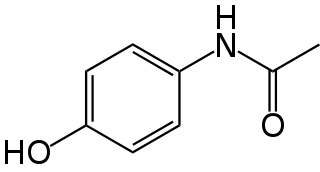
Paracetamol, also known as acetaminophen, is a medication used to treat fever and mild to moderate pain. At a standard dose, paracetamol only slightly decreases body temperature; it is inferior to ibuprofen in that respect, and the benefits of its use for fever are unclear. Paracetamol may relieve pain in acute mild migraine but only slightly in episodic tension headache. However, the aspirin/paracetamol/caffeine combination helps with both conditions where the pain is mild and is recommended as a first-line treatment for them. Paracetamol is effective for post-surgical pain, but it is inferior to ibuprofen. The paracetamol/ibuprofen combination provides further increase in potency and is superior to either drug alone. The pain relief paracetamol provides in osteoarthritis is small and clinically insignificant. The evidence in its favor for the use in low back pain, cancer pain and neuropathic pain is insufficient.
An international nonproprietary name (INN) is an official generic and non-proprietary name given to a pharmaceutical drug or an active ingredient. INNs are intended to make communication more precise by providing a unique standard name for each active ingredient, to avoid prescribing errors. The INN system has been coordinated by the World Health Organization (WHO) since 1953.
Oxycodone/paracetamol, sold under the brand name Percocet among others, is a combination of the opioid oxycodone with paracetamol (acetaminophen), used to treat moderate to severe pain.

Amoxicillin/clavulanic acid, also known as co-amoxiclav or amox-clav, is an antibiotic medication used for the treatment of a number of bacterial infections. It is a combination consisting of amoxicillin, a β-lactam antibiotic, and potassium clavulanate, a β-lactamase inhibitor. It is specifically used for otitis media, streptococcal pharyngitis, pneumonia, cellulitis, urinary tract infections, and animal bites. It is taken by mouth or by injection into a vein.

Dihydrocodeine is a semi-synthetic opioid analgesic prescribed for pain or severe dyspnea, or as an antitussive, either alone or compounded with paracetamol (acetaminophen) or aspirin. It was developed in Germany in 1908 and first marketed in 1911.

Dextropropoxyphene is an analgesic in the opioid category, patented in 1955 and manufactured by Eli Lilly and Company. It is an optical isomer of levopropoxyphene. It is intended to treat mild pain and also has antitussive and local anaesthetic effects. The drug has been taken off the market in Europe and the US due to concerns of fatal overdoses and heart arrhythmias. It is still available in Australia, albeit with restrictions after an application by its manufacturer to review its proposed banning. Its onset of analgesia is said to be 20–30 minutes and peak effects are seen about 1.5–2.0 hours after oral administration.
A United States Adopted Name (USAN) is a unique nonproprietary name assigned to a medication marketed in the United States. Each name is assigned by the USAN Council, which is co-sponsored by the American Medical Association (AMA), the United States Pharmacopeial Convention (USP), and the American Pharmacists Association (APhA).
The British Pharmacopoeia (BP) is the national pharmacopoeia of the United Kingdom. It is an annually published collection of quality standards for medicinal substances in the UK, which is used by individuals and organisations involved in pharmaceutical research, development, manufacture and testing.

Metamizole, or dipyrone, is a painkiller, spasm reliever, and fever reliever that also has anti-inflammatory effects. It is most commonly given by mouth or by intravenous infusion.
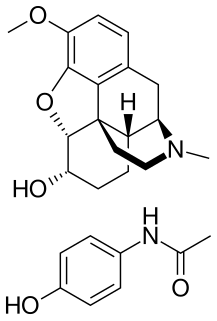
Co-dydramol (BAN) is a non-proprietary name used to denote a particular compound analgesic, a combination of dihydrocodeine tartrate and paracetamol. Co-dydramol tablets are used for the relief of moderate pain. Co-dydramol is part of a series of combination drugs available in the UK and other countries including co-codaprin.
Propyphenazone/paracetamol/caffeine is an analgesic combination indicated for the management of headache. It contains the analgesics propyphenazone and paracetamol and the stimulant caffeine.

Hydrocodone/paracetamol is the combination of the pain medications hydrocodone and paracetamol (acetaminophen). It is used to treat moderate to severe pain. It is taken by mouth. Recreational use is common in the United States.

Codeine is an opiate and prodrug of morphine used to treat pain, coughing, and diarrhea. It is found naturally in the sap of the opium poppy, Papaver somniferum. It is typically used to treat mild to moderate degrees of pain. Greater benefit may occur when combined with paracetamol (acetaminophen) or a nonsteroidal anti-inflammatory drug (NSAID) such as aspirin or ibuprofen. Evidence does not support its use for acute cough suppression in children or adults. In Europe, it is not recommended as a cough medicine in those under 12 years of age. It is generally taken by mouth. It typically starts working after half an hour, with maximum effect at two hours. Its effects last for about four to six hours. Codeine exhibits abuse potential similar to other opioid medications.

Levallorphan, also known as levallorphan tartrate (USAN), is an opioid modulator of the morphinan family used as an opioid analgesic and opioid antagonist/antidote. It acts as an antagonist of the μ-opioid receptor (MOR) and as an agonist of the κ-opioid receptor (KOR), and as a result, blocks the effects of stronger agents with greater intrinsic activity such as morphine whilst simultaneously producing analgesia.
Drug nomenclature is the systematic naming of drugs, especially pharmaceutical drugs. In the majority of circumstances, drugs have 3 types of names: chemical names, the most important of which is the IUPAC name; generic or nonproprietary names, the most important of which are the International Nonproprietary Names (INNs); and trade names, which are brand names. Under the INN system, generic names for drugs are constructed out of affixes and stems that classify the drugs into useful categories while keeping related names distinguishable. A marketed drug might also have a company code or compound code.
Oxycodone/ibuprofen is an oral combination drug formulation of the opioid analgesic oxycodone and the nonsteroidal anti-inflammatory drug (NSAID) ibuprofen that is used in the treatment of chronic and acute pain. This particular drug is supplied in a fixed dose combination tablet which contains Oxycodone Hydrochloride, USP 5 mg with Ibuprofen, USP 400 mg.
References
- ↑ "Paracetamol - different name in USA?". irishhealth.com. Archived from the original on 4 December 2014. Retrieved 4 December 2014.
- ↑ Mr Anthony C Cartwright (28 June 2015). The British Pharmacopoeia, 1864 to 2014: Medicines, International Standards and the State. Ashgate Publishing, Ltd. pp. 155–. ISBN 978-1-4724-2032-9.
- ↑ "Triclofos". Drugs.com. Retrieved 15 November 2019.
- ↑ "2001/82/EC"
- ↑ "2001/83/EC"
- ↑ "2003/63/EC"
- ↑ Aronson, JK (19 February 2000). ""Where name and image meet"--the argument for "adrenaline"". BMJ (Clinical Research Ed.). 320 (7233): 506–9. doi:10.1136/bmj.320.7233.506. PMC 1127537 . PMID 10678871.