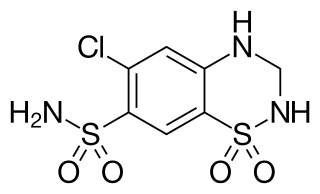
Hydrochlorothiazide, sold under the brand name Hydrodiuril among others, is a diuretic medication used to treat hypertension and swelling due to fluid build-up. Other uses include treating diabetes insipidus and renal tubular acidosis and to decrease the risk of kidney stones in those with a high calcium level in the urine. Hydrochlorothiazide is taken by mouth and may be combined with other blood pressure medications as a single pill to increase effectiveness. Hydrochlorothiazide is a thiazide medication which inhibits reabsorption of sodium and chloride ions from the distal convoluted tubules of the kidneys, causing a natriuresis. This initially increases urine volume and lowers blood volume. It is believed to reduce peripheral vascular resistance.

Amiloride, sold under the trade name Midamor among others, is a medication typically used with other medications to treat high blood pressure or swelling due to heart failure or cirrhosis of the liver. Amiloride is classified as a potassium-sparing diuretic. Amiloride is often used together with another diuretic, such as a thiazide or loop diuretic. It is taken by mouth. Onset of action is about two hours and it lasts for about a day.
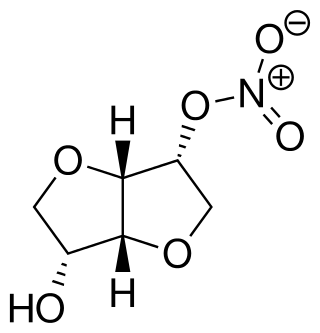
Isosorbide mononitrate, sold under many brand names, is a medication used for heart-related chest pain (angina), heart failure and esophageal spasms. It can be used both to treat and to prevent heart-related chest pain; however, it is generally less preferred than beta blockers or calcium channel blockers. It is taken by mouth.

Lisinopril is a medication belonging to the drug class of angiotensin-converting enzyme (ACE) inhibitors and is used to treat high blood pressure, heart failure, and heart attacks. For high blood pressure it is usually a first-line treatment. It is also used to prevent kidney problems in people with diabetes mellitus. Lisinopril is taken by mouth. Full effect may take up to four weeks to occur.

Triamterene is a potassium-sparing diuretic often used in combination with thiazide diuretics for the treatment of high blood pressure or swelling. The combination with hydrochlorothiazide, is known as hydrochlorothiazide/triamterene.

Benazepril, sold under the brand name Lotensin among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease. It is a reasonable initial treatment for high blood pressure. It is taken by mouth. Versions are available as the combinations benazepril/hydrochlorothiazide and benazepril/amlodipine.

Losartan, sold under the brand name Cozaar among others, is a medication used to treat high blood pressure (hypertension). It is in the angiotensin receptor blocker (ARB) family of medication, and is considered protective of the kidneys. Besides hypertension, it is also used in diabetic kidney disease, heart failure, and left ventricular enlargement. It comes as a tablet that is taken by mouth. It may be used alone or in addition to other blood pressure medication. Up to six weeks may be required for the full effects to occur.

Olmesartan, sold under the brand name Benicar among others, is a medication used to treat high blood pressure (hypertension). It is taken by mouth. Versions are available as the combination olmesartan/hydrochlorothiazide and olmesartan/amlodipine.

Isradipine is a calcium channel blocker of the dihydropyridine class. It is usually prescribed for the treatment of high blood pressure in order to reduce the risk of stroke and heart attack.

Lubiprostone, sold under the brand name Amitiza among others, is a medication used in the management of chronic idiopathic constipation, predominantly irritable bowel syndrome-associated constipation in women and opioid-induced constipation. The drug is owned by Mallinckrodt and is marketed by Takeda Pharmaceutical Company.

Mephentermine is a cardiac stimulant.

Paricalcitol (chemically it is 19-nor-1,25-(OH)2-vitamin D2. Marketed by Abbott Laboratories under the trade name Zemplar) is a drug used for the prevention and treatment of secondary hyperparathyroidism (excessive secretion of parathyroid hormone) associated with chronic kidney failure. It is an analog of 1,25-dihydroxyergocalciferol, the active form of vitamin D2 (ergocalciferol).

In the United States the National Collegiate Athletic Association (NCAA), has since the 1970s been patrolling the usage of illegal drugs and substances for student-athletes attending universities and colleges. In 1999, NCAA Drug Committee published a list containing substances banned for the usage to student-athletes. Year after year it is updated and given to those students participating in college sports. If any student is caught taking any of the substances, they are subjected to suspension or even banned from participating in NCAA sports and possibly attending the university.
Losartan/hydrochlorothiazide, sold under the brand name Hyzaar among others, is a fixed-dose combination medication used to treat high blood pressure when losartan is not sufficient. It consists of losartan and hydrochlorothiazide. It is taken by mouth.

Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is an anti-cancer medication used to treat medullary thyroid cancer, renal cell carcinoma, and hepatocellular carcinoma. It is a small molecule inhibitor of the tyrosine kinases c-Met and VEGFR2, and also inhibits AXL and RET. It was discovered and developed by Exelixis Inc.
Emtricitabine/rilpivirine/tenofovir is a fixed-dose combination of antiretroviral drugs for the treatment of HIV/AIDS. The drug was co-developed by Gilead Sciences and Johnson & Johnson's Tibotec division and was approved by the Food and Drug Administration in August 2011, and by the European Medicines Agency in November 2011, for patients who have not previously been treated for HIV. It is available as a once-a-day single tablet.













