Calcium channel blockers (CCB), calcium channel antagonists or calcium antagonists are a group of medications that disrupt the movement of calcium through calcium channels. Calcium channel blockers are used as antihypertensive drugs, i.e., as medications to decrease blood pressure in patients with hypertension. CCBs are particularly effective against large vessel stiffness, one of the common causes of elevated systolic blood pressure in elderly patients. Calcium channel blockers are also frequently used to alter heart rate, to prevent peripheral and cerebral vasospasm, and to reduce chest pain caused by angina pectoris.
Ryanodine receptors form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons. There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.

Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system's ability to readily detoxify the reactive intermediates or to repair the resulting damage. Disturbances in the normal redox state of cells can cause toxic effects through the production of peroxides and free radicals that damage all components of the cell, including proteins, lipids, and DNA. Oxidative stress from oxidative metabolism causes base damage, as well as strand breaks in DNA. Base damage is mostly indirect and caused by the reactive oxygen species generated, e.g., O−
2, OH and H2O2. Further, some reactive oxidative species act as cellular messengers in redox signaling. Thus, oxidative stress can cause disruptions in normal mechanisms of cellular signaling.

Calcium/calmodulin-dependent protein kinase type IV is an enzyme that in humans is encoded by the CAMK4 gene.

Synaptotagmin-1 is a protein that in humans is encoded by the SYT1 gene.

In enzymology, a trypanothione synthase (EC 6.3.1.9) is an enzyme that catalyzes the chemical reaction

Alpha-actinin-1 is a protein that in humans is encoded by the ACTN1 gene.

Calsenilin is a protein that in humans is encoded by the KCNIP3 gene.

Calcium/calmodulin-dependent protein kinase type 1 is an enzyme that in humans is encoded by the CAMK1 gene.

Kv channel-interacting protein 2 also known as KChIP2 is a protein that in humans is encoded by the KCNIP2 gene.

Peroxiredoxin-6 is a protein that in humans is encoded by the PRDX6 gene. It is a member of the peroxiredoxin family of antioxidant enzymes.

Chloride channel accessory 1 is a protein that in humans is encoded by the CLCA1 gene.

Sarcoplasmic/endoplasmic reticulum calcium ATPase 3 is an enzyme that in humans is encoded by the ATP2A3 gene.

Voltage-dependent L-type calcium channel subunit beta-4 is a protein that in humans is encoded by the CACNB4 gene.

Chloride channel accessory 2 is a protein that in humans is encoded by the CLCA2 gene.

Inositol 1,4,5-trisphosphate receptor, type 3, also known as ITPR3, is a protein which in humans is encoded by the ITPR3 gene. The protein encoded by this gene is both a receptor for inositol triphosphate and a calcium channel.

Anoctamin-1 (ANO1), also known as Transmembrane member 16A (TMEM16A), is a protein that, in humans, is encoded by the ANO1 gene. Anoctamin-1 is a voltage-gated calcium-activated anion channel, which acts as a chloride channel and a bicarbonate channel. additionally Anoctamin-1 is apical iodide channel. It is expressed in smooth muscle, epithelial cells, vomeronasal neurons, olfactory sustentacular cells, and is highly expressed in interstitial cells of Cajal (ICC) throughout the gastrointestinal tract.

BRL-32872 is an experimental drug candidate that provides a novel approach to the treatment of cardiac arrhythmia. Being a derivative of verapamil, it possesses the ability to inhibit Ca+2 membrane channels. Specific modifications in hydrogen bonding activity, nitrogen lone pair availability, and molecular flexibility allow BRL-32872 to inhibit K+ channels as well. As such, BRL-32872 is classified as both a class III (K+ blocking) and class IV (Ca+2 blocking) antiarrhythmic agent.
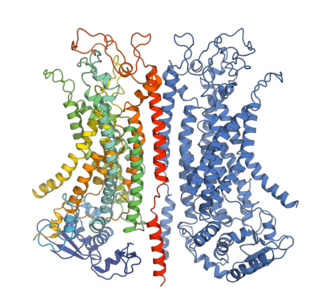
The Calcium-Dependent Chloride Channel (Ca-ClC) proteins (or calcium-activated chloride channels, are heterogeneous groups of ligand-gated ion channels for chloride that have been identified in many epithelial and endothelial cell types as well as in smooth muscle cells. They include proteins from several structurally different families: chloride channel accessory, bestrophin, and calcium-dependent chloride channel anoctamin channels ANO1 is highly expressed in human gastrointestinal interstitial cells of Cajal, which are proteins which serve as intestinal pacemakers for peristalsis. In addition to their role as chloride channels some CLCA proteins function as adhesion molecules and may also have roles as tumour suppressors. These eukaryotic proteins are "required for normal electrolyte and fluid secretion, olfactory perception, and neuronal and smooth muscle excitability" in animals. Members of the Ca-CIC family are generally 600 to 1000 amino acyl residues in length and exhibit 7 to 10 transmembrane segments.

ML-SI3 is a chemical compound which acts as an "antagonist" of the TRPML family of calcium channels, with greatest activity at the TRPML1 channel, although it also blocks the related TRPML2 and TRPML3 channels with lower affinity. It is used for research into the role of TRPML1 and its various functions in lysosomes and elsewhere in the body.

















