 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anbesol, Lanacane, Orajel, others |
| AHFS/Drugs.com | |
| Routes of administration | Topical, Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.094 |
| Chemical and physical data | |
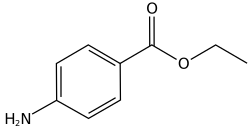
| Formula | C9H11NO2 |
| Molar mass | 165.192 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Benzocaine, sold under the brand name Orajel amongst others, is a local anesthetic, belonging to the amino ester drug class, commonly used as a topical painkiller or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments such as products for oral ulcers. It is combined with antipyrine to form A/B ear drops. In the US, products containing benzocaine for oral application are contraindicated in children younger than two years old. [2] [3]
Contents
- Medical uses
- Other uses
- Available forms
- Side effects
- Chemistry
- Synthesis
- History
- Society and culture
- Veterinary uses
- References
It was first synthesised in 1890 in Germany and approved for medical use in 1902. [4]
