
Procaine is a local anesthetic drug of the amino ester group. It is most commonly used in dental procedures to numb the area around a tooth and is also used to reduce the pain of intramuscular injection of penicillin. Owing to the ubiquity of the trade name Novocain or Novocaine, in some regions, procaine is referred to generically as novocaine. It acts mainly as a sodium channel blocker. Today, it is used therapeutically in some countries due to its sympatholytic, anti-inflammatory, perfusion-enhancing, and mood-enhancing effects.
Chloroethane, commonly known as ethyl chloride, is a chemical compound with chemical formula CH3CH2Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor.

Cyclopentolate is a muscarinic antagonist. It is commonly used as an eye drop during pediatric eye examinations to dilate the eye (mydriatic) and prevent the eye from focusing/accommodating (cycloplegic). Cyclopentolate or atropine can also be administered to reverse muscarinic and central nervous system effects of indirect cholinomimetic (anti-AChase) administration.
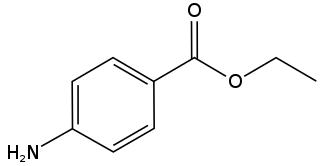
Benzocaine, sold under the brand name Orajel amongst others, is a local anesthetic, belonging to the amino ester drug class, commonly used as a topical painkiller or in cough drops. It is the active ingredient in many over-the-counter anesthetic ointments such as products for oral ulcers. It is combined with antipyrine to form A/B ear drops. In the US, products containing benzocaine for oral application are contraindicated in children younger than two years old. In the European Union, the contraindication applies to children under 12 years of age.

Fischer esterification or Fischer–Speier esterification is a special type of esterification by refluxing a carboxylic acid and an alcohol in the presence of an acid catalyst. The reaction was first described by Emil Fischer and Arthur Speier in 1895. Most carboxylic acids are suitable for the reaction, but the alcohol should generally be primary or secondary. Tertiary alcohols are prone to elimination. Contrary to common misconception found in organic chemistry textbooks, phenols can also be esterified to give good to near quantitative yield of products. Commonly used catalysts for a Fischer esterification include sulfuric acid, p-toluenesulfonic acid, and Lewis acids such as scandium(III) triflate. For more valuable or sensitive substrates other, milder procedures such as Steglich esterification are used. The reaction is often carried out without a solvent or in a non-polar solvent to facilitate the Dean-Stark method. Typical reaction times vary from 1–10 hours at temperatures of 60-110 °C.

Ethyl acetate is the organic compound with the formula CH3CO2CH2CH3, simplified to C4H8O2. This colorless liquid has a characteristic sweet smell and is used in glues, nail polish removers, and in the decaffeination process of tea and coffee. Ethyl acetate is the ester of ethanol and acetic acid; it is manufactured on a large scale for use as a solvent.
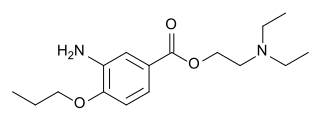
Proxymetacaine (INN) or proparacaine (USAN) is a topical anesthetic drug of the aminoester group.
A topical anesthetic is a local anesthetic that is used to numb the surface of a body part. They can be used to numb any area of the skin as well as the front of the eyeball, the inside of the nose, ear or throat, the anus and the genital area. Topical anesthetics are available in creams, ointments, aerosols, sprays, lotions, and jellies. Examples include benzocaine, butamben, dibucaine, lidocaine, oxybuprocaine, pramoxine, proxymetacaine (proparacaine), and tetracaine.

A corneal ulcer, or ulcerative keratitis, is an inflammatory condition of the cornea involving loss of its outer layer. It is very common in dogs and is sometimes seen in cats. In veterinary medicine, the term corneal ulcer is a generic name for any condition involving the loss of the outer layer of the cornea, and as such is used to describe conditions with both inflammatory and traumatic causes.

Butamben is a local anesthetic. Proprietary names includes Alvogil in Spain and Alvogyl in Switzerland. It is one of three components in the topical anesthetic Cetacaine.

Vernal keratoconjunctivitis is a recurrent, bilateral, and self-limiting type of conjunctivitis having a periodic seasonal incidence.

Loteprednol is a topical corticosteroid used to treat inflammations of the eye. It is marketed by Bausch and Lomb as Lotemax and Loterex.
Pramocaine is a topical anesthetic discovered at Abbott Laboratories in 1953 and used as an antipruritic. During research and development, pramocaine hydrochloride stood out among a series of alkoxy aryl alkamine ethers as an especially good topical local anesthetic agent. Pharmacologic study revealed it to be potent and of low acute and subacute toxicity, well tolerated by most mucous membranes and of a low sensitizing index in humans. Like other local anesthetics, pramocaine decreases the permeability of neuronal membranes to sodium ions, blocking both initiation and conduction of nerve impulses. Depolarization and repolarization of excitable neural membranes is thus inhibited, leading to numbness.

Corneal ulcer, also called keratitis, is an inflammatory or, more seriously, infective condition of the cornea involving disruption of its epithelial layer with involvement of the corneal stroma. It is a common condition in humans particularly in the tropics and in farming. In developing countries, children afflicted by vitamin A deficiency are at high risk for corneal ulcer and may become blind in both eyes persisting throughout life. In ophthalmology, a corneal ulcer usually refers to having an infection, while the term corneal abrasion refers more to a scratch injury.

Quinisocaine (INN) or dimethisoquin is a topical anesthetic used as an antipruritic.

Efaroxan is an α2-adrenergic receptor antagonist and antagonist of the imidazoline receptor.

Butacaine is a white crystalline ester used as a local anesthetic.
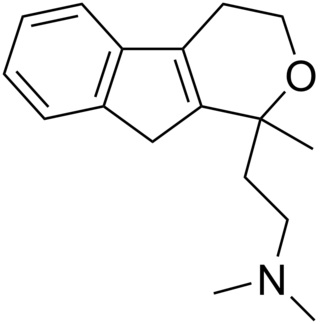
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI). It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed. Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.
Cetacaine is a topical anesthetic that contains the active ingredients benzocaine (14%), butamben (2%), and tetracaine hydrochloride (2%). Cetacaine also contains small amounts of benzalkonium chloride at 0.5% and 0.005% of cetyl dimethyl ethyl ammonium bromide all in a bland water-soluble base. Although Cetacaine has been widely used in the medical and dental fields, it has yet to be officially approved by the FDA. Cetacaine is produced by the company Cetylite Industries, Inc. and they provide Cetacaine in three forms: liquid, gel, and spray.

Phenaglycodol is a drug described as a tranquilizer or sedative which has anxiolytic and anticonvulsant properties. It is related pharmacologically to meprobamate, though it is not a carbamate.


















