 | |
| Clinical data | |
|---|---|
| Trade names | Ethmozine |
| Other names | Moricizine (USAN US) |
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a601214 |
| Pregnancy category |
|
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | 34–38% |
| Protein binding | 95% |
| Elimination half-life | 3–4 hours (healthy volunteers), 6–13 hours (cardiac disease) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.046.216 |
| Chemical and physical data | |
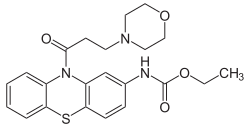
| Formula | C22H25N3O4S |
| Molar mass | 427.52 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Moracizine [1] or moricizine, sold under the trade name Ethmozine, is an antiarrhythmic of class IC. [2] It was used for the prophylaxis and treatment of serious and life-threatening ventricular arrhythmias, [3] but was withdrawn in 2007 for commercial reasons. [4]
