| |
 | |
| Clinical data | |
|---|---|
| Trade names | Gilurytmal, Ritmos, Aritmina |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.022.219 |
| Chemical and physical data | |
| Formula | C20H26N2O2 |
| Molar mass | 326.440 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
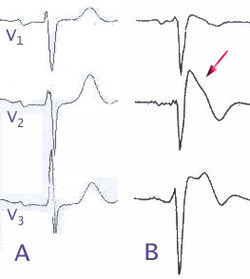
Ajmaline (also known by trade names Gilurytmal, Ritmos, and Aritmina) is an alkaloid that is classified as a 1-A antiarrhythmic agent. It is often used to induce arrhythmic contraction in patients suspected of having Brugada syndrome. Individuals suffering from Brugada syndrome will be more susceptible to the arrhythmogenic effects of the drug, and this can be observed on an electrocardiogram as an ST elevation.
Contents
The compound was first isolated by Salimuzzaman Siddiqui in 1931 [1] from the roots of Rauvolfia serpentina . He named it ajmaline, after Hakim Ajmal Khan, one of the most illustrious practitioners of Unani medicine in South Asia. [2] Ajmaline can be found in most species of the genus Rauvolfia as well as Catharanthus roseus . [3] In addition to Southeast Asia, Rauvolfia species have also been found in tropical regions of India, Africa, South America, and some oceanic islands. Other indole alkaloids found in Rauvolfia include reserpine, ajmalicine, serpentine, corynanthine, and yohimbine. While 86 alkaloids have been discovered throughout Rauvolfia vomitoria , ajmaline is mainly isolated from the stem bark and roots of the plant. [3]
Due to the low bioavailability of ajmaline, a semisynthetic propyl derivative called prajmaline (trade name Neo-gilurythmal) was developed that induces effects similar to those of its predecessor but which has better bioavailability and absorption. [4]