
An action potential occurs when the membrane potential of a specific cell rapidly rises and falls. This depolarization then causes adjacent locations to similarly depolarize. Action potentials occur in several types of animal cells, called excitable cells, which include neurons, muscle cells, and in some plant cells. Certain endocrine cells such as pancreatic beta cells, and certain cells of the anterior pituitary gland are also excitable cells.
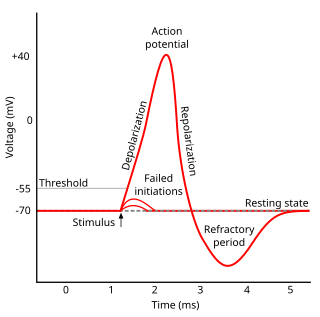
Refractoriness is the fundamental property of any object of autowave nature not responding to stimuli, if the object stays in the specific refractory state. In common sense, refractory period is the characteristic recovery time, a period that is associated with the motion of the image point on the left branch of the isocline .

In electrocardiography, the ventricular cardiomyocyte membrane potential is about −90 mV at rest, which is close to the potassium reversal potential. When an action potential is generated, the membrane potential rises above this level in five distinct phases.
- Phase 4: Resting membrane potential remains stable at ≈−90 mV.
- Phase 0: Rapid depolarisation, shifting the voltage to positive. Specialised membrane proteins in the cell membrane selectively allow sodium ions to enter the cell. This causes the membrane potential to rise at a rate of about 300 V/s. As the membrane voltage rises sodium channels close due to a process called inactivation.
- Phase 1: Rapid repolarisation.
- Phase 2: Plateau, the longest phase, approximately 100ms.
- Phase 3: Rapid repolarisation, which returns the membrane potential to resting potential.
Hyperpolarization is a change in a cell's membrane potential that makes it more negative. It is the opposite of a depolarization. It inhibits action potentials by increasing the stimulus required to move the membrane potential to the action potential threshold.
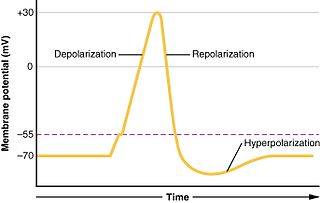
In biology, depolarization or hypopolarization is a change within a cell, during which the cell undergoes a shift in electric charge distribution, resulting in less negative charge inside the cell compared to the outside. Depolarization is essential to the function of many cells, communication between cells, and the overall physiology of an organism.

Membrane potential is the difference in electric potential between the interior and the exterior of a biological cell. That is, there is a difference in the energy required for electric charges to move from the internal to exterior cellular environments and vice versa, as long as there is no acquisition of kinetic energy or the production of radiation. The concentration gradients of the charges directly determine this energy requirement. For the exterior of the cell, typical values of membrane potential, normally given in units of milli volts and denoted as mV, range from –80 mV to –40 mV.
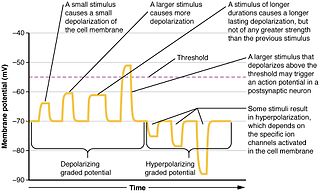
Graded potentials are changes in membrane potential that vary according to the size of the stimulus, as opposed to being all-or-none. They include diverse potentials such as receptor potentials, electrotonic potentials, subthreshold membrane potential oscillations, slow-wave potential, pacemaker potentials, and synaptic potentials. The magnitude of a graded potential is determined by the strength of the stimulus. They arise from the summation of the individual actions of ligand-gated ion channel proteins, and decrease over time and space. They do not typically involve voltage-gated sodium and potassium channels, but rather can be produced by neurotransmitters that are released at synapses which activate ligand-gated ion channels. They occur at the postsynaptic dendrite in response to presynaptic neuron firing and release of neurotransmitter, or may occur in skeletal, smooth, or cardiac muscle in response to nerve input. These impulses are incremental and may be excitatory or inhibitory.

The cardiac conduction system transmits the signals generated by the sinoatrial node – the heart's pacemaker, to cause the heart muscle to contract, and pump blood through the body's circulatory system. The pacemaking signal travels through the right atrium to the atrioventricular node, along the bundle of His, and through the bundle branches to Purkinje fibers in the walls of the ventricles. The Purkinje fibers transmit the signals more rapidly to stimulate contraction of the ventricles.

The cardiac action potential is a brief change in voltage across the cell membrane of heart cells. This is caused by the movement of charged atoms between the inside and outside of the cell, through proteins called ion channels. The cardiac action potential differs from action potentials found in other types of electrically excitable cells, such as nerves. Action potentials also vary within the heart; this is due to the presence of different ion channels in different cells.
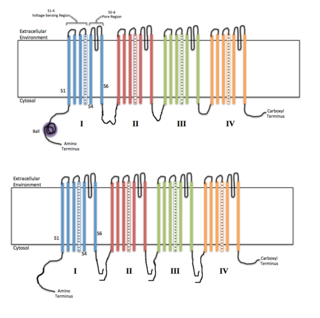
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between the sides of the cell membrane.
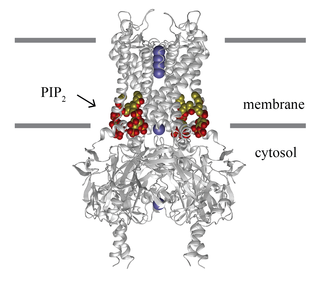
Inward-rectifier potassium channels (Kir, IRK) are a specific lipid-gated subset of potassium channels. To date, seven subfamilies have been identified in various mammalian cell types, plants, and bacteria. They are activated by phosphatidylinositol 4,5-bisphosphate (PIP2). The malfunction of the channels has been implicated in several diseases. IRK channels possess a pore domain, homologous to that of voltage-gated ion channels, and flanking transmembrane segments (TMSs). They may exist in the membrane as homo- or heterooligomers and each monomer possesses between 2 and 4 TMSs. In terms of function, these proteins transport potassium (K+), with a greater tendency for K+ uptake than K+ export. The process of inward-rectification was discovered by Denis Noble in cardiac muscle cells in 1960s and by Richard Adrian and Alan Hodgkin in 1970 in skeletal muscle cells.

Potassium voltage-gated channel subfamily E member 1 is a protein that in humans is encoded by the KCNE1 gene.
Stromatoxin is a spider toxin that blocks certain delayed-rectifier and A-type voltage-gated potassium channels.

Potassium voltage-gated channel, Shab-related subfamily, member 1, also known as KCNB1 or Kv2.1, is a protein that, in humans, is encoded by the KCNB1 gene.

Potassium voltage-gated channel, Isk-related family, member 3 (KCNE3), also known as MinK-related peptide 2(MiRP2) is a protein that in humans is encoded by the KCNE3 gene.
Low-threshold spikes (LTS) refer to membrane depolarizations by the T-type calcium channel. LTS occur at low, negative, membrane depolarizations. They often follow a membrane hyperpolarization, which can be the result of decreased excitability or increased inhibition. LTS result in the neuron reaching the threshold for an action potential. LTS is a large depolarization due to an increase in Ca2+ conductance, so LTS is mediated by calcium (Ca2+) conductance. The spike is typically crowned by a burst of two to seven action potentials, which is known as a low-threshold burst. LTS are voltage dependent and are inactivated if the cell's resting membrane potential is more depolarized than −60mV. LTS are deinactivated, or recover from inactivation, if the cell is hyperpolarized and can be activated by depolarizing inputs, such as excitatory postsynaptic potentials (EPSP). LTS were discovered by Rodolfo Llinás and coworkers in the 1980s.
Cellular neuroscience is a branch of neuroscience concerned with the study of neurons at a cellular level. This includes morphology and physiological properties of single neurons. Several techniques such as intracellular recording, patch-clamp, and voltage-clamp technique, pharmacology, confocal imaging, molecular biology, two photon laser scanning microscopy and Ca2+ imaging have been used to study activity at the cellular level. Cellular neuroscience examines the various types of neurons, the functions of different neurons, the influence of neurons upon each other, and how neurons work together.
In electrophysiology, the term gating refers to the opening (activation) or closing of ion channels. This change in conformation is a response to changes in transmembrane voltage.

The cardiac transient outward potassium current (referred to as Ito1 or Ito ) is one of the ion currents across the cell membrane of heart muscle cells. It is the main contributing current during the repolarizing phase 1 of the cardiac action potential. It is a result of the movement of positively charged potassium (K+) ions from the intracellular to the extracellular space. Ito1 is complemented with Ito2 resulting from Cl− ions to form the transient outward current Ito.

Guangxitoxin, also known as GxTX, is a peptide toxin found in the venom of the tarantula Plesiophrictus guangxiensis. It primarily inhibits outward voltage-gated Kv2.1 potassium channel currents, which are prominently expressed in pancreatic β-cells, thus increasing insulin secretion.















