 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Glucotrol, Glucotrol XL, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a684060 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Sulfonylurea |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 100% (regular formulation) 90% (extended release) |
| Protein binding | 98 to 99% |
| Metabolism | Liver hydroxylation |
| Elimination half-life | 2 to 5 hours |
| Excretion | Kidney and fecal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.919 |
| Chemical and physical data | |
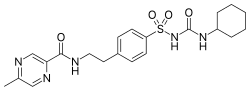
| Formula | C21H27N5O4S |
| Molar mass | 445.54 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 to 209 °C (406 to 408 °F) |
| |
| |
| | |
Glipizide, sold under the brand name Glucotrol among others, is an anti-diabetic medication of the sulfonylurea class used to treat type 2 diabetes. [1] [2] It is used together with a diabetic diet and exercise. [1] [2] It is not indicated for use by itself in type 1 diabetes. [1] [2] It is taken by mouth. [1] [2] Effects generally begin within half an hour and can last for up to a day. [1]
Contents
Common side effects include nausea, diarrhea, low blood sugar, and headache. [1] Other side effects include sleepiness, skin rash, and shakiness. [3] The dose may need to be adjusted in those with liver or kidney disease. [1] Use during pregnancy or breastfeeding is not recommended. [3] It works by stimulating the pancreas to release insulin and increases tissue sensitivity to insulin. [1]
Glipizide was approved for medical use in the United States in 1984. [1] It is available as a generic medication. [1] In 2023, it was the 42nd most commonly prescribed medication in the United States, with more than 15 million prescriptions. [4] [5]
