| |
 | |
| Clinical data | |
|---|---|
| Trade names | Glucobay, Precose, Prandase |
| Other names | (2R,3R,4R,5S,6R)-5-{[(2R,3R,4R,5S,6R)-5- {[(2R,3R,4S,5S,6R)-3,4-dihydroxy-6-methyl- 5-{[(1S,4R,5S,6S)-4,5,6-trihydroxy-3- (hydroxymethyl)cyclohex-2-en-1-yl]amino} tetrahydro-2H-pyran-2-yl]oxy}-3,4-dihydroxy- 6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}- 6-(hydroxymethyl)tetrahydro-2H-pyran-2,3,4-triol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a696015 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Extremely low |
| Metabolism | Gastrointestinal tract |
| Elimination half-life | 2 hours |
| Excretion | Kidney (less than 2%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.054.555 |
| Chemical and physical data | |
| Formula | C25H43NO18 |
| Molar mass | 645.608 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Acarbose (INN) [1] [2] is an anti-diabetic drug used to treat diabetes mellitus type 2 and, in some countries, prediabetes. It is a generic sold in Europe and China as Glucobay (Bayer AG), in North America as Precose (Bayer Pharmaceuticals), and in Canada as Prandase (Bayer AG).
Contents
- Medical uses
- Efficacy
- Combination therapy
- Adverse effects
- Pharmacology
- Mechanism of action
- Metabolism
- Natural distribution
- In molecular biology
- Research
- References
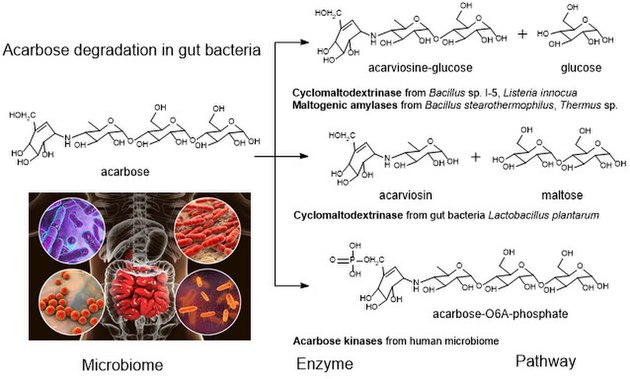
Acarbose is a starch blocker. It works by inhibiting alpha glucosidase, an intestinal enzyme that releases glucose from larger carbohydrates such as starch and sucrose. It is composed of an acarviosin moiety with a maltose at the reducing terminus. It can be degraded by a number of gut bacteria. [3]
Acarbose is cheap and popular in China, but not in the U.S. One physician explains that use in the U.S. is limited because it is not potent enough to justify the side effects of diarrhea and flatulence. [4] However, a large study concluded in 2013 that "acarbose is effective, safe and well tolerated in a large cohort of Asian patients with type 2 diabetes." [5] A possible explanation for the differing opinions is an observation that acarbose is significantly more effective in patients eating a relatively high-starch Eastern diet. [6]