The following is a glossary of diabetes which explains terms connected with diabetes.

Diabetic ketoacidosis (DKA) is a potentially life-threatening complication of diabetes mellitus. Signs and symptoms may include vomiting, abdominal pain, deep gasping breathing, increased urination, weakness, confusion and occasionally loss of consciousness. A person's breath may develop a specific "fruity" smell. The onset of symptoms is usually rapid. People without a previous diagnosis of diabetes may develop DKA as the first obvious symptom.
Hyperglycemia or Hyperglycaemia is a condition in which an excessive amount of glucose circulates in the blood plasma. This is generally a blood sugar level higher than 11.1 mmol/L (200 mg/dL), but symptoms may not start to become noticeable until even higher values such as 13.9–16.7 mmol/L (~250–300 mg/dL). A subject with a consistent fasting blood glucose range between ~5.6 and ~7 mmol/L is considered slightly hyperglycemic, and above 7 mmol/L is generally held to have diabetes. For diabetics, glucose levels that are considered to be too hyperglycemic can vary from person to person, mainly due to the person's renal threshold of glucose and overall glucose tolerance. On average, however, chronic levels above 10–12 mmol/L (180–216 mg/dL) can produce noticeable organ damage over time.

Diabetic coma is a life-threatening but reversible form of coma found in people with diabetes mellitus.

Glipizide, sold under the brand name Glucotrol among others, is an anti-diabetic medication of the sulfonylurea class used to treat type 2 diabetes. It is used together with a diabetic diet and exercise. It is not indicated for use by itself in type 1 diabetes. It is taken by mouth. Effects generally begin within half an hour and can last for up to a day.
Drugs used in diabetes treat diabetes mellitus by decreasing glucose levels in the blood. With the exception of insulin, most GLP-1 receptor agonists, and pramlintide, all diabetes medications are administered orally and are thus called oral hypoglycemic agents or oral antihyperglycemic agents. There are different classes of hypoglycemic drugs, and selection of the appropriate agent depends on the nature of diabetes, age, and situation of the person, as well as other patient factors.
Diabetes is a chronic disease in cats whereby either insufficient insulin response or insulin resistance leads to persistently high blood glucose concentrations. Diabetes affects up to 1 in 230 cats, and may be becoming increasingly common. Diabetes is less common in cats than in dogs. The condition is treatable, and if treated properly the cat can experience a normal life expectancy. In cats with type 2 diabetes, prompt effective treatment may lead to diabetic remission, in which the cat no longer needs injected insulin. Untreated, the condition leads to increasingly weak legs in cats and eventually to malnutrition, ketoacidosis and/or dehydration, and death.

Type 1 diabetes (T1D), formerly known as juvenile diabetes, is an autoimmune disease that originates when cells that make insulin are destroyed by the immune system. Insulin is a hormone required for the cells to use blood sugar for energy and it helps regulate glucose levels in the bloodstream. It results in high blood sugar levels in the body prior to treatment. The common symptoms of this elevated blood sugar are frequent urination, increased thirst, increased hunger, weight loss, and other serious complications. Additional symptoms may include blurry vision, tiredness, and slow wound healing. Symptoms typically develop over a short period of time, often a matter of weeks if not months.
An insulin analog is any of several types of medical insulin that are altered forms of the hormone insulin, different from any occurring in nature, but still available to the human body for performing the same action as human insulin in terms of controlling blood glucose levels in diabetes. Through genetic engineering of the underlying DNA, the amino acid sequence of insulin can be changed to alter its ADME characteristics. Officially, the U.S. Food and Drug Administration (FDA) refers to these agents as insulin receptor ligands, although they are usually just referred to as insulin analogs or even just insulin.
Neutral Protamine Hagedorn (NPH) insulin, also known as isophane insulin, is an intermediate-acting insulin given to help control blood sugar levels in people with diabetes. The words refer to neutral pH, protamine a protein, and Hans Christian Hagedorn the insulin researcher who invented this formulation. It is designed to improve the delivery of insulin, and is one of the earliest examples of engineered drug delivery.
Hyperosmolar hyperglycemic state (HHS), also known as hyperosmolar non-ketotic state (HONK), is a complication of diabetes mellitus in which high blood sugar results in high osmolarity without significant ketoacidosis. Symptoms include signs of dehydration, weakness, leg cramps, vision problems, and an altered level of consciousness. Onset is typically over days to weeks. Complications may include seizures, disseminated intravascular coagulopathy, mesenteric artery occlusion, or rhabdomyolysis.
Insulin detemir, sold under the brand name Levemir among others, is a long-acting modified form of medical insulin used to treat both type 1 and type 2 diabetes. It is used by injection under the skin. It is effective for up to 24 hours.

Insulin aspart, sold under the brand name NovoLog, among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is generally used by injection under the skin but may also be used by injection into a vein. Maximum effect occurs after about 1–3 hours and lasts for 3–5 hours. Generally a longer-acting insulin like insulin NPH is also needed.

Insulin lispro, sold under the brand name Humalog among others, is a modified type of medical insulin used to treat type 1 and type 2 diabetes. It is delivered subcutaneously either by injection or from an insulin pump. Onset of effects typically occurs within 30 minutes and lasts about 5 hours. Often a longer-acting insulin like insulin NPH is also needed.
As a medication, insulin is any pharmaceutical preparation of the protein hormone insulin that is used to treat high blood glucose. Such conditions include type 1 diabetes, type 2 diabetes, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. Insulin is also used along with glucose to treat hyperkalemia. Typically it is given by injection under the skin, but some forms may also be used by injection into a vein or muscle. There are various types of insulin, suitable for various time spans. The types are often all called insulin in the broad sense, although in a more precise sense, insulin is identical to the naturally occurring molecule whereas insulin analogues have slightly different molecules that allow for modified time of action. It is on the World Health Organization's List of Essential Medicines. In 2021, it was the 179th most commonly prescribed medication in the United States, with more than 2 million prescriptions.
Complications of diabetes are secondary diseases that are a result of elevated blood glucose levels that occur in diabetic patients. These complications can be divided into two types: acute and chronic. Acute complications are complications that develop rapidly and can be exemplified as diabetic ketoacidosis (DKA), hyperglycemic hyperosmolar state (HHS), lactic acidosis (LA), and hypoglycemia. Chronic complications develop over time and are generally classified in two categories: microvascular and macrovascular. Microvascular complications include neuropathy, nephropathy, and retinopathy; while cardiovascular disease, stroke, and peripheral vascular disease are included in the macrovascular complications.
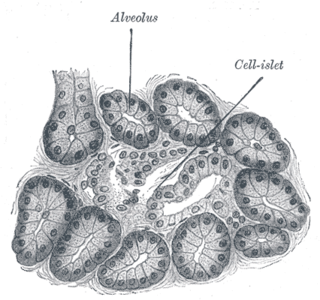
Diabetes mellitus is a disease in which the beta cells of the endocrine pancreas either stop producing insulin or can no longer produce it in enough quantity for the body's needs. The disease can affect humans as well as animals such as dogs.

Lente insulin was an intermediate duration insulin that is no longer used in humans. The onset of lente insulin is one to two hours after the dose is administered, and the peak effect is approximately 8 to 12 hours after administration, with some effects lasting over 24 hours.
Ultralente insulin was a long-acting form of insulin. It has an onset of 4 to 6 hours, a peak of 14 to 24 hours, and a duration of 28 to 36 hours. Ultralente insulin, along with lente insulin, were discontinued in the US by manufacturers in the mid-2000s. One of the reasons for discontinuation was declining use in favor of NPH insulin and other newer insulin products. The FDA withdrew approval for ultralente insulin products by 2011.
Ketosis-prone diabetes (KPD) is an intermediate form of diabetes that has some characteristics of type 1 and some of type 2 diabetes. Type 1 diabetes involves autoimmune destruction of pancreatic beta cells which create insulin. This occurs earlier in a person's life, leading to patients being insulin dependent, and the lack of natural insulin makes patients prone to a condition called diabetic ketoacidosis (DKA). Type 2 diabetes is different in that it is usually caused by insulin resistance in the body in older patients leading to beta cell burnout over time, and is not prone to DKA. KPD is a condition that involves DKA like type 1, but occurs later in life and can regain beta cell function like type 2 diabetes. However, it is distinct from latent autoimmune diabetes of adults (LADA), a form of type 1 sometimes referred to as type 1.5 that does not occur with DKA. There are also distinctions to be made between KPD and LADA as patients who exhibit KPD symptoms can regain beta cell function similar to type 2 diabetics whereas LADA will not exhibit this reclamation of beta cell function.










